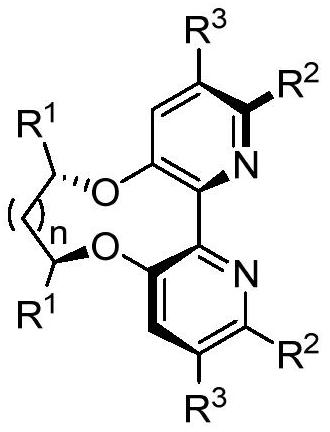
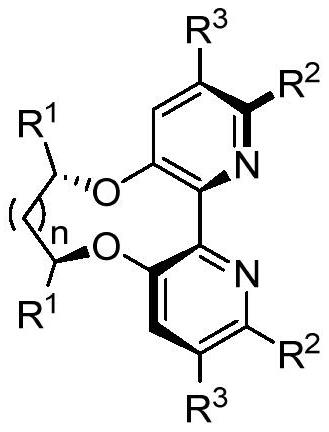
Bipyridine ligands with axial chirality and synthesis method thereof
一种联吡啶类、合成方法的技术,应用在具有轴手性的联吡啶配体及其合成领域,能够解决应用鲜有报道、吡啶配体发展少等问题,达到反应步骤少、收率高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of bipyridine ligand 4
[0043]
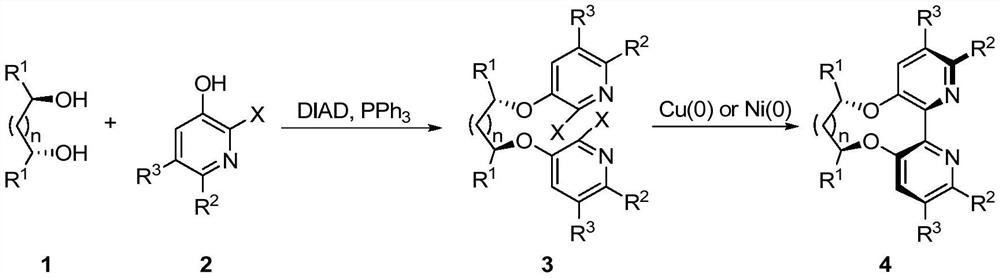
[0044] Add diol 1 (2.080g, 20mmol), pyridine 2 (9.72g, 44mmol), and triphenylphosphine (12.6g, 48mmol) into a 250mL reaction flask under nitrogen protection, and add 80mL tetrahydrofuran at 0°C and stir to dissolve. DIAD (9.70 g, 48 mmol) was dissolved in 20 mL of tetrahydrofuran solution dropwise from the constant pressure dropping funnel. After the dropwise addition, the system was an orange-red transparent solution. After 28 hours of reaction, only a small amount of raw materials remained, and the reaction was stopped.
[0045]First spin dry THF, add 20mL dichloromethane to dissolve, add a large amount of petroleum ether, until a large amount of solids precipitate (mostly the reduction product of triphenoxyphosphine and DIAD. If it cannot be precipitated, scrape the bottle wall repeatedly with a small spoon), pump Filtrate (wash the solid with petroleum ether several times during suction filtration, ch...
Embodiment 2
[0047] Embodiment 2: the synthesis of bipyridine ligand 8
[0048]
[0049] Add pyridine 5 (6.458g, 60mmol) to tetrahydrofuran / water (50 / 50mL) mixed solution, stir to dissolve and then add iodine (16.8g, 66mmol) and sodium bicarbonate (5.281g, 66mmol) to the reaction flask, at room temperature After 3 days of reaction, the reaction solution was purple-black, and solids were precipitated. Add 10% sodium thiosulfate solution until the purple-black color disappeared, and a large amount of white solids precipitated. 8.19 g of off-white solids were obtained by suction filtration, which was verified as product 6 by NMR.
[0050] Add diol (2.080g, 20mmol), pyridine 6 (10.3g, 44mmol), and triphenylphosphine (12.6g, 48mmol) into a 250mL reaction flask under nitrogen protection, and add 80mL tetrahydrofuran at 0°C and stir to dissolve. DIAD (9.70 g, 48 mmol) was dissolved in 20 mL of tetrahydrofuran solution dropwise from the constant pressure dropping funnel. After the dropwise addi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com