Gliclazide sustained-release tablet dissolution curve determination method, similarity evaluation method and application thereof
A technology of gliclazide and a determination method, which is applied in the field of determination of the dissolution curve of gliclazide sustained-release tablets, can solve the problem that the movement state of the mechanical device is single, the gap is large, and the in vivo release situation of the sustained-release preparation cannot be better simulated, etc. problems, to achieve consistent evaluation, a wide range of dosage forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Determination of the dissolution profile of Gliclazide sustained-release tablets by "basket method"
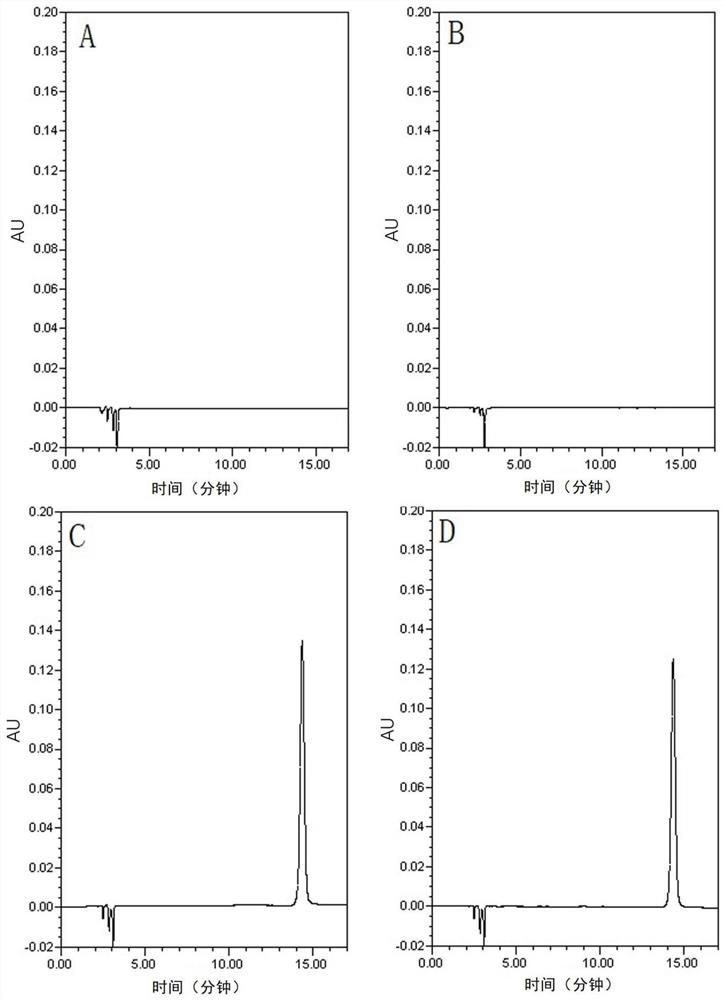
[0047] 1.1. Specificity test
[0048] Test group:
[0049] Blank solvent: use acetonitrile-water (volume ratio 45:55) as the blank solvent, shake well, and filter to obtain.
[0050] Blank excipients: prepare the blank excipients without gliclazide according to the prescription ratio of gliclazide sustained-release tablets, weigh the blank excipients equivalent to the weight of 1 tablet, put them in a 100mL measuring bottle, add 45mL of acetonitrile, ultrasonically dissolve, and dilute with water To the mark, shake well, filter, accurately measure 5mL of the filtrate, put it in a 25mL measuring bottle, dilute with acetonitrile-water (45:55 by volume) to the mark, shake well, and you get it.
[0051] Reference substance solution: take an appropriate amount of gliclazide reference substance, weigh it accurately, add acetonitrile to dissolve, and dilute with w...
Embodiment 2
[0072] Example 2: Determination of the dissolution profile of Gliclazide Sustained-release Tablets with a "flow cell closed-loop system"
[0073] The flow cell method (FTC) is divided into two types: closed-loop and open-loop. The open-loop or closed-loop flow cells adopt commercial instruments, which are composed of medium storage bottles, constant flow piston pumps, temperature-controlled flow cells, filters, sampling systems and samples. Collector composition.
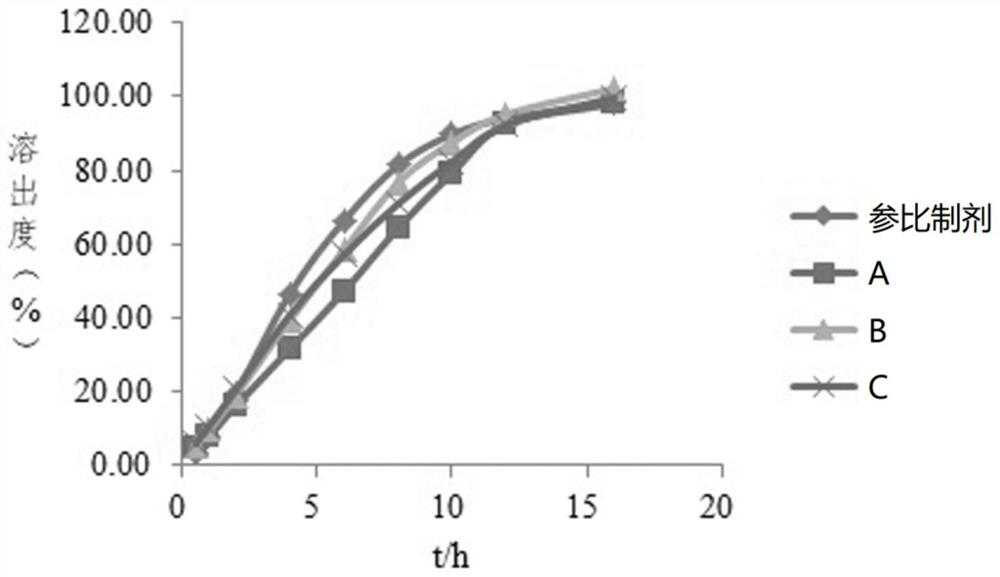
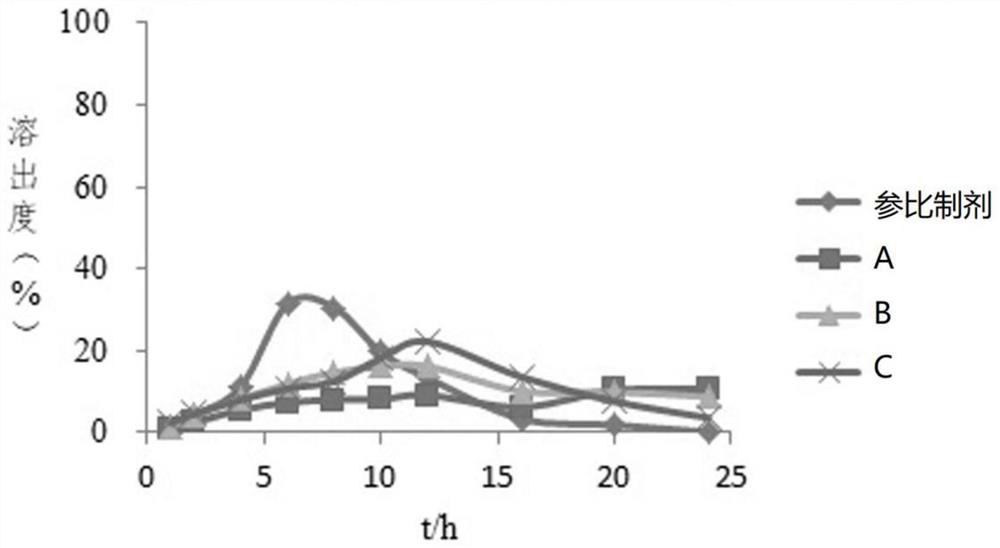
[0074] The reference preparation described in Example 1 and the imitation preparations of three manufacturers (imitation preparation A, imitation preparation B, and imitation preparation C) were selected as samples, and the closed-loop flow cell was used to dissolve the gliclazide sustained-release tablets. The specific steps were as follows:
[0075] The sample is placed on the specially designed sample rack in the flow cell. The dissolution medium passes through the water bath of the heat exchanger equipped with a...
Embodiment 3
[0095] Example 3: Determination of the dissolution profile of Gliclazide Sustained Release Tablets with "flow cell open loop system"
[0096] The reference preparation described in Example 1 and the generic preparations of three manufacturers (generic preparation A, generic preparation B, and generic preparation C) were selected as samples, and an open-loop flow cell was used to dissolve the gliclazide sustained-release tablets. The specific steps were as follows :
[0097] Place the sample on a specially designed sample rack in the flow cell, and the dissolution medium passes through the flow cell, so that a large amount of fresh dissolution medium passes through the sample continuously, so that the sample is in contact with the fresh dissolution medium at any time, and the main drug components are gradually dissolved;
[0098] The conical part of the flow cell is filled with glass beads with a particle size of 1 mm, so that the dissolution medium flows in a laminar flow in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Medium volume | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com