Plasmid system
A technology of plasmids and vector plasmids, applied in the direction of using vectors to introduce foreign genetic material, viral peptides, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0567] Example 1 - Construction ('trans-resolution') of helper and carrier plasmids for a two-plasmid system
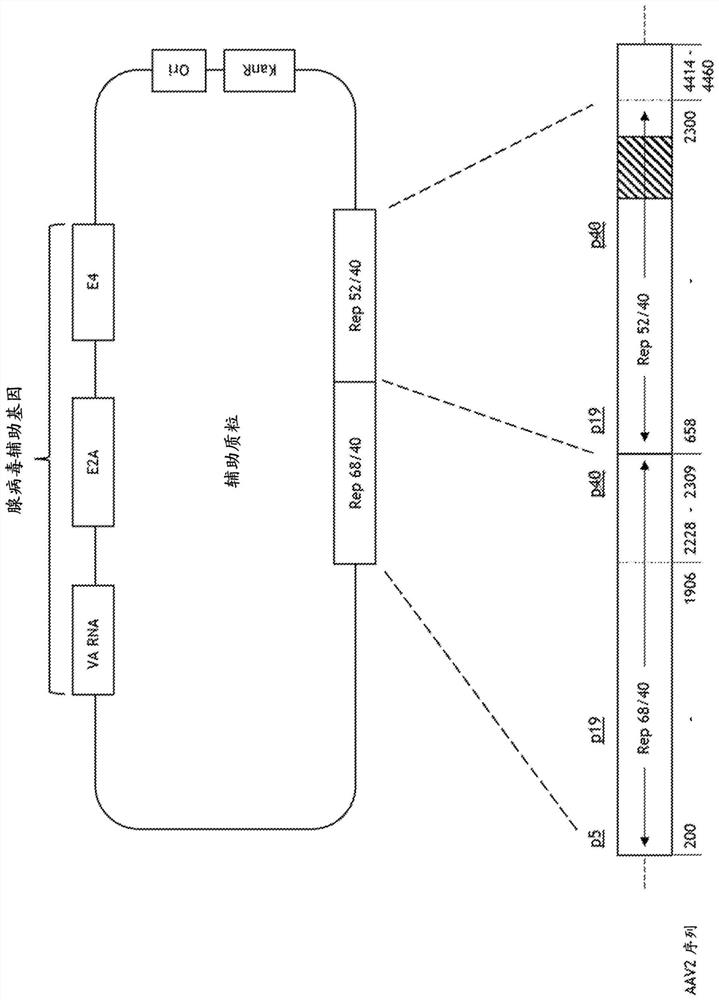
[0568] helper plasmid
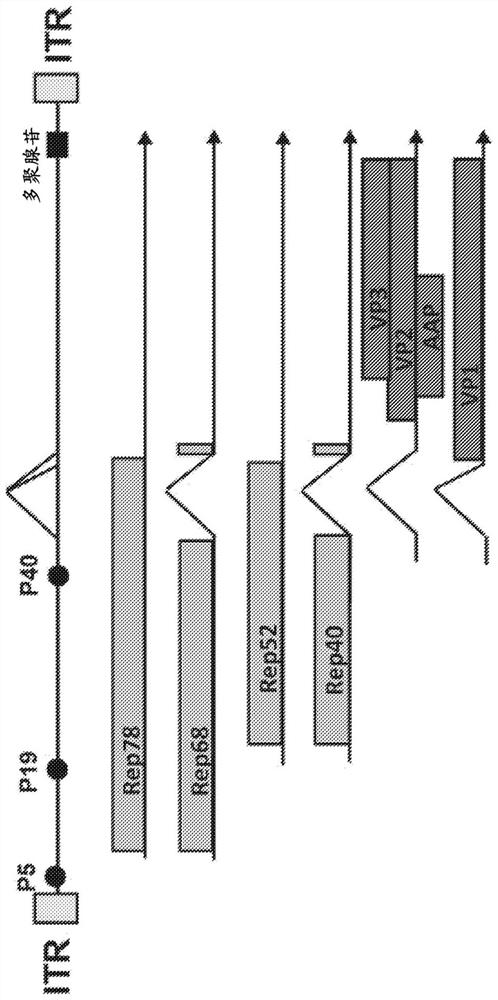
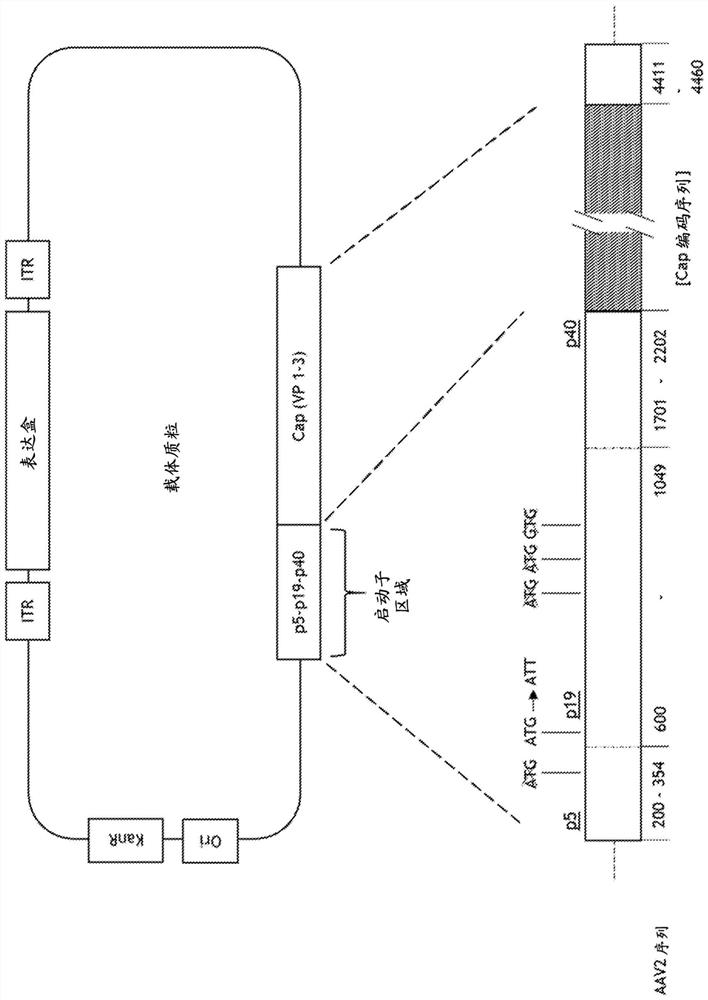
[0569] Nucleotides 200-4497 of wild-type AAV2 (Genbank Accession No. AF043303; SEQ ID NO: 1 ) containing the rep and cap genes were cloned into pUC19 to prepare a helper plasmid (Yanisch-Perron et al (1985), Gene, 33: 103-119). Next, AAV2 nucleotides 4461-4497 were deleted to minimize sequence homology to the vector plasmid (the construction of which is described below). To prevent Rep 78 expression while maintaining Rep 68 expression, an intron within the cloned rep gene was deleted. To provide expression of Rep52 following intronic deletion, the AAV2 nucleotide corresponding to rep 52 containing the p19 promoter was cloned immediately 3' to the intronless rep 68 gene. Most of the cap gene sequence was then deleted.
[0570] Ablation of the TATA box (T corresponding to AAV2 position 1823 and mutation of AAG corresponding to AAV2 position...
Embodiment 2
[0579] Example 2 - Two-plasmid System: Comparison of Helper Plasmid:Vector Plasmid Ratio
[0580] cell culture
[0581] Under standard conditions at 37°C, 95% relative humidity and 5% v / v CO 2 conditions, supplemented with 10% fetal bovine serum (FBS) and 1% GlutaMax TM HEK293T cells were maintained by adherent culture in Duchenne's modified Eagle's medium (DMEM) (L-alanine-L-glutamine dipeptide). Cell confluency during passaging ranged from 40-95%.
[0582] Transfection of HEK293T cells and preparation of cell lysates
[0583] Different molar plasmid ratios (from helper plasmid:vector plasmid 3:1 to 1:6) were used with helper plasmid and carrier plasmid (the latter containing the engineered cap gene and Factor IX expression cassette; Example 1) while maintaining total plasmid The amount of DNA transfected into HEK293T cells. The day before transfection, 1.5 × 10 per square centimeter of culture area 5 live cells were seeded in a 6 cm dish in a volume of 3 ml DMEM, 10%...
Embodiment 3
[0599] Example 3 - Determination of replication-competent AAV (rcAAV) in rAAV produced by the trans-split two-plasmid system frequency
[0600] rcAAV test
[0601] will use Purified drug substance from rAAV, which was mass-produced in bioreactor cells transfected with the two-plasmid system and purified using a number of downstream processes to remove product impurities, was limit tested for the presence of rcAAV. Using an engineered cap gene and (i) α-galactosidase A expression cassette (as described in Example 1) or (ii) except for a different partial codon-optimized Factor IX coding sequence as in Example The same Factor IX expression cassette vector plasmid as mentioned in 1 was used to produce rAAV batches. The two plasmid systems used to produce this rAAV batch used a shortened vector plasmid backbone and a corrected helper plasmid sequence as mentioned in Examples 1 and 2, respectively. In this limit test, rAAV transduced HEK293 cells in its most concentrated fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com