Systemic delivery of serum stable plasmid lipid particles for cancer therapy
a systemic delivery and cancer technology, applied in the field of tumors in mammals, can solve the problems of rapid degradation of naked dna in the blood, non-satisfactory criteria, and the use of systemic delivery systems, and achieve the effects of improving the properties or characteristics of formulations, reducing toxicities, and encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
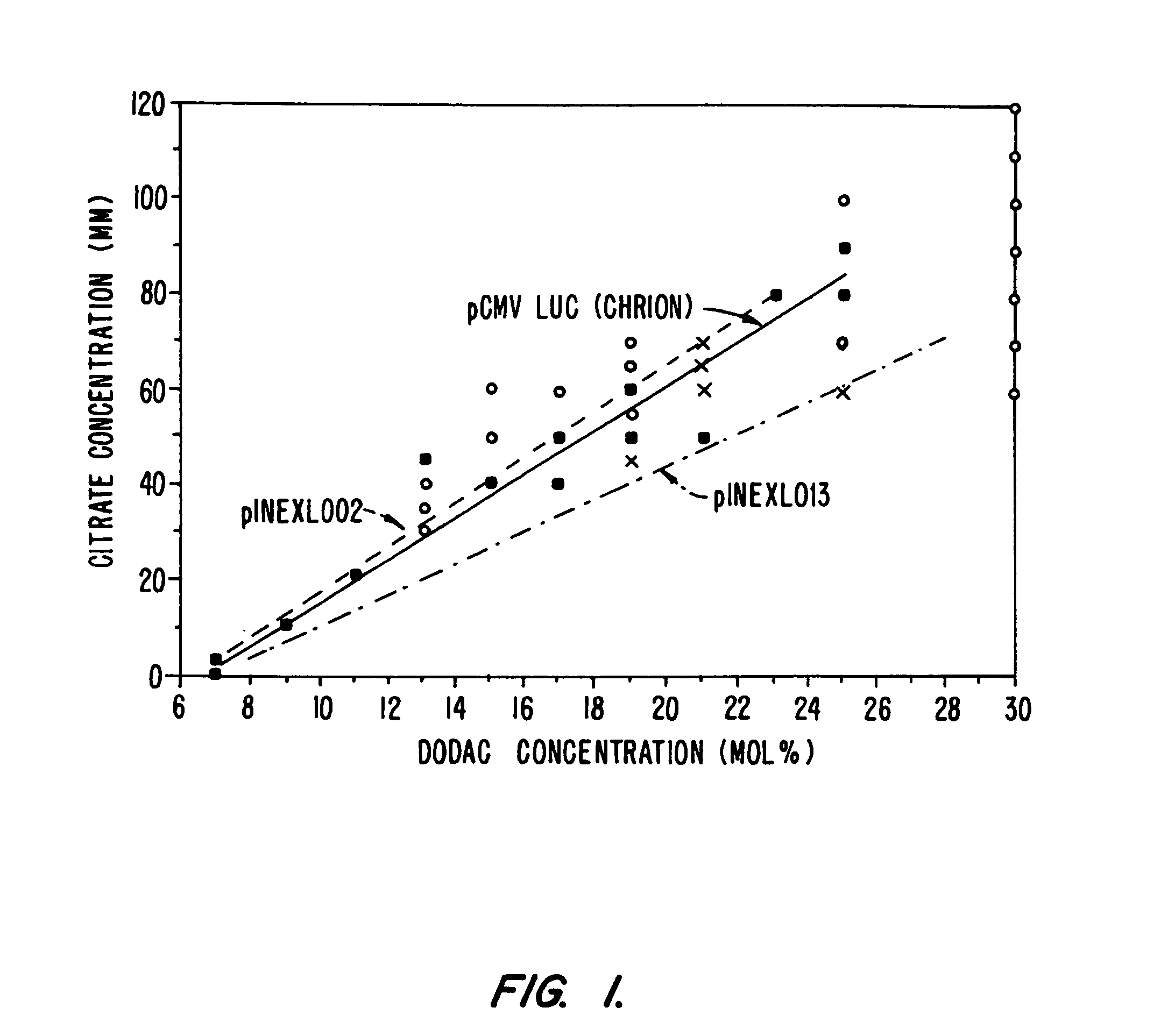
[0093] This example illustrates the synthesis of 5 lipid-plasmid particle formulations for systemic delivery.
[0094] Materials: Plasmids are preferably supercoiled, 4000 to 15000 bp in length, encoding genes and enhancer elements, etc. as desired. Cationic lipid, N,N-dioleyl-N,N-dimethyl ammonium chloride (“DODAC”) and monomethoxy polyethylene2000 glycol succinate-(C8:0-ceramide) (“PEG-Cer-C8”) were synthesized at Inex Pharmaceuticals Corp. Dioleyl-phosphatidylethanolamine (DOPE) was supplied by Northern Lipids, Vancouver. Standard dialysis membranes: Spectro / Por 5 regenerated Cellulose (12-14,000 MWCO) was purchased from VWR (Manufactured by Spectrum Medical Industries Inc.). Sodium Citrate was purchased from BDH. Sodium Chloride, Triton X-100 and Octyl-beta-D-glucopyranoside (“OGP”) were obtained from VWR Scientific, Fisher Scientific or Sigma Chemical Company.
Formulation 1.1 (Or, Alternatively, INEX 303 or INEX 303i)
[0095] Plasmid (50-400 μg) is incubated with DODAC in 500 μL ...
example 2
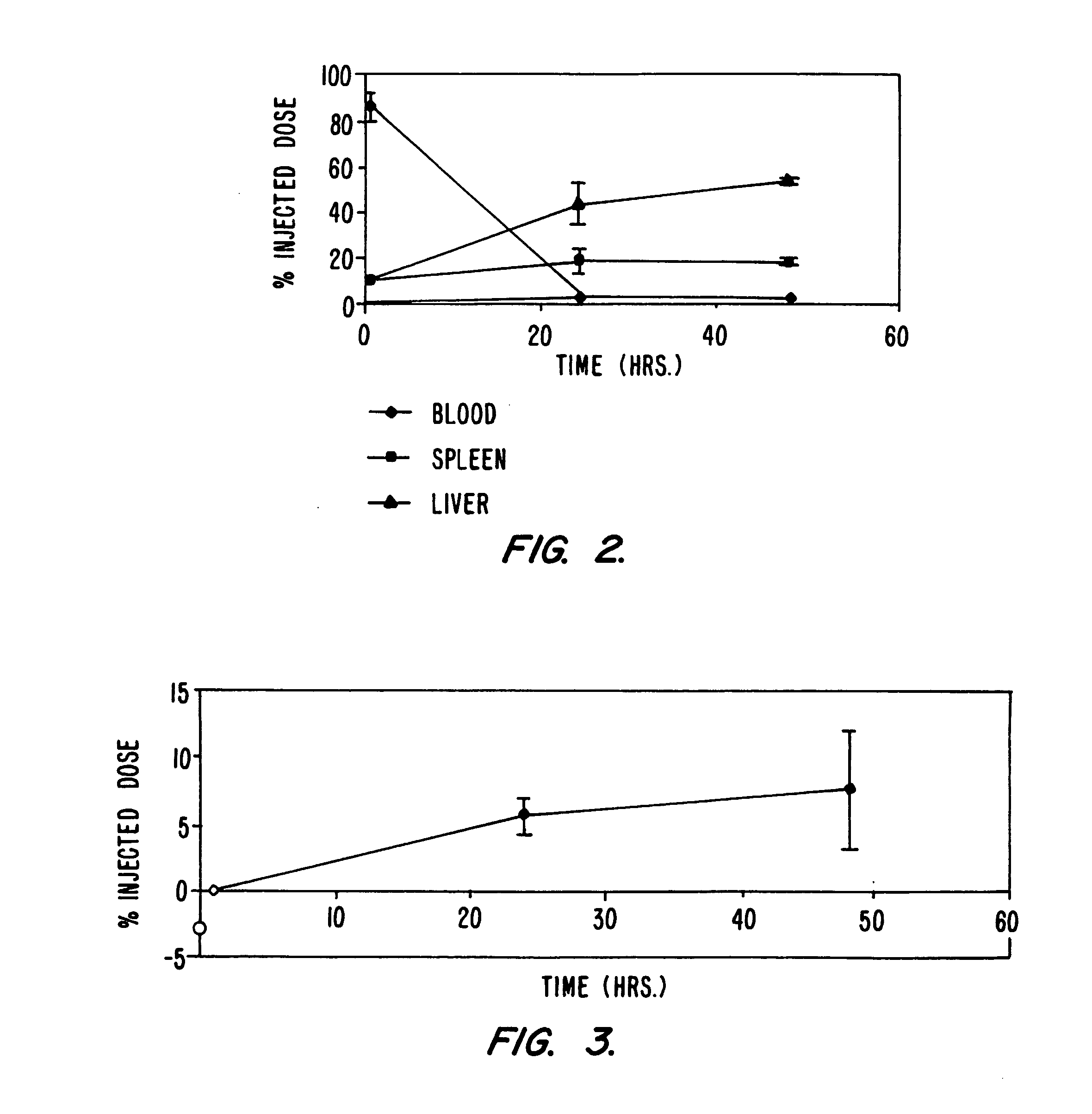
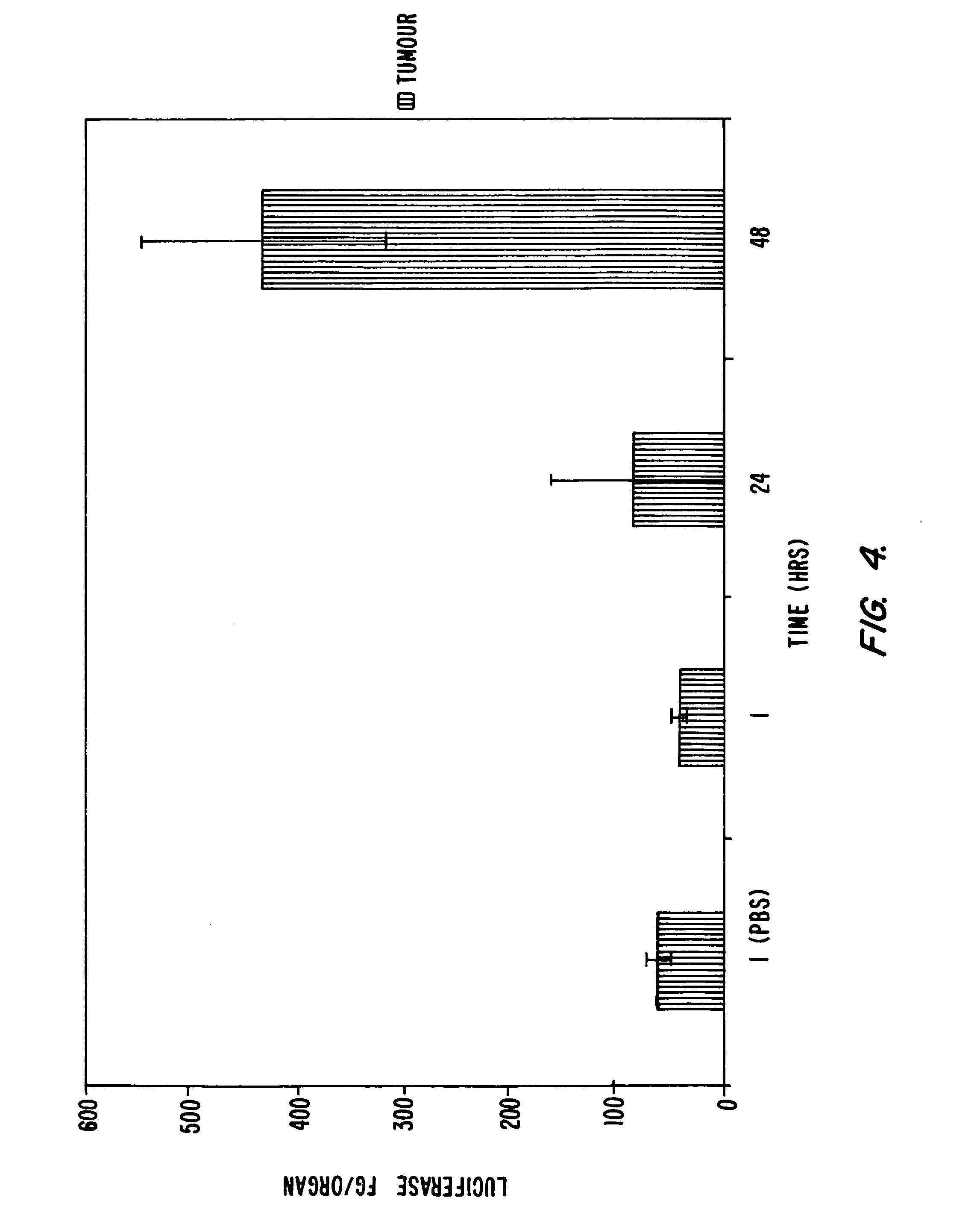
[0128] This example illustrates the measurement of the therapeutic effect of lipid formulated ganciclovir on subcutaneous tumors transfected with lipid encapsulated HSV-TK.
Mice perGroupTumorPlasmidProdrugRouteAssayGroupAB16L018PBSIVTumor Volume6 C57BB16L018GCVIVTumor Volume6 C57CB16pTK010PBSIVTumor Volume6 C57DB16pTK010GCVIVTumor Volume6 C57
[0129] On day zero, 24 female C57 mice (Harlan Sprague Dawley, Inc., Indianapolis, Ind.) are seeded sub-cutaneously with 100,000 B16 mice melanoma cells (NCI catalog B 16BL-6) in a total volume of 50 μL (groups A, B, C, D). Tumor volume is determined daily by measuring the length, width and height of the tumor with skin calipers as soon as possible and every day thereafter. Groups A to D are treated with 100 μg plasmid of the appropriate lipid-formulated plasmid, formulated according to Example 1, once daily beginning at 9:00 a.m. on day five and on every day following. The plasmid formulation is injected IV in the tail vein in a total volume o...
example 5
[0132] This example sets forth the protocol for stable transfection of B16 tumor cells with HSV-TK, for use in Examples 6 and 7, as described in Short Protocols in Molecular Biology, Third Edition, page 9-13 to 9-15, with the following modifications.
[0133] According to the method, the following materials were used. Plasmids: pCMVTKIRESneo is based on plasmid pBR322 and includes a CMV promoter, HSV-TK gene, internal ribosome entry site and neomycin resistance gene. L018 is also based on pBR322, but carrying a CMV promoter and a luciferase gene. First, plate B 16 murine melanoma cells in a tissue culture flask (T-75) at 5×105 cells / flask in 10 mL MEM media with addition of 10% FBS and Glutamine and grow overnight in CO2 incubator at 37° C. to 70% confluency. Next, aspirate media and feed cells with 3.8 mL fresh media per flask 2 h prior to transfection and prepare plasmid / lipid Lipofectin (GIBCO BRL) aggregate in a polystyrene tube according to manufacturer's instructions. Alternativ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com