Application of imidazole propionic acid as biomarker for predicting diabetic nephropathy and detection kit
A technology of biomarkers and detection kits, applied in the field of diagnosis, can solve problems such as inability to give treatment, inability to detect diseases early, and lack of effective early diagnosis indicators for DKD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The technical solutions and beneficial effects of the present invention will be further described below in conjunction with specific embodiments.
[0020] patient choice
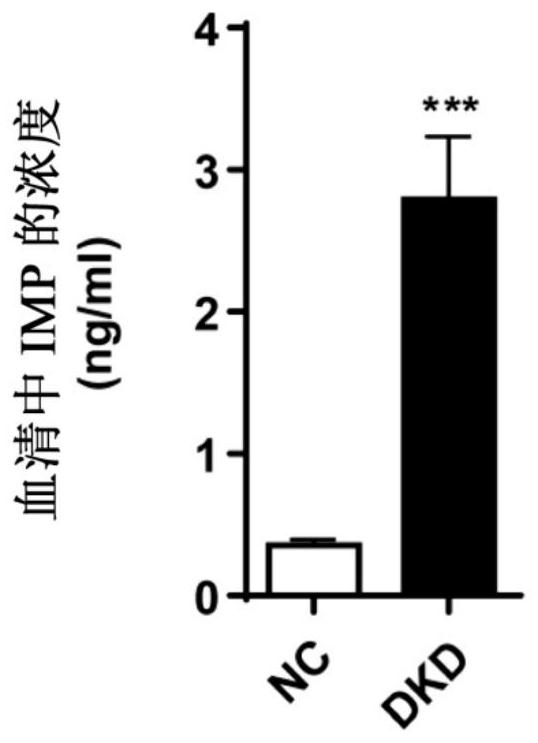
[0021] From December 2019 to December 2020, 16 patients with diabetic nephropathy admitted to the Department of Endocrinology, The First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine were selected as the observation group; 12 healthy volunteers were recruited as the control group.
[0022] It should be noted that the "Chinese Clinical Guidelines for the Prevention and Treatment of Diabetic Kidney Disease (2019 Edition)" and "Expert Consensus on the Prevention and Treatment of Diabetic Kidney Disease (2014 Edition)" were used as the clinical diagnosis basis for diabetic kidney disease. The patient's estimated glomerular filtration rate (eGFR) is less than 60ml·min -1 ·1.73m -2 Or urine albumin / creatinine ratio (UACR) higher than 30mg / g for more than 3 months can be diagnos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com