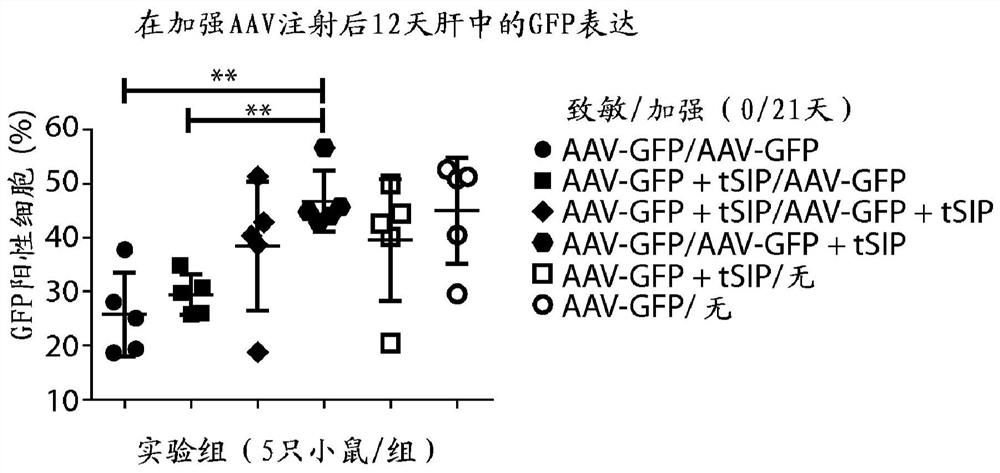
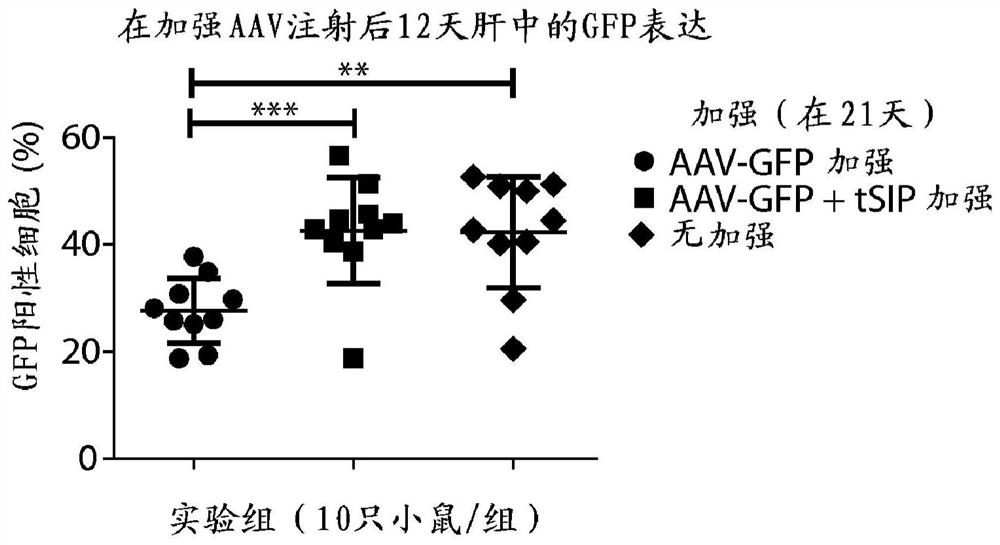
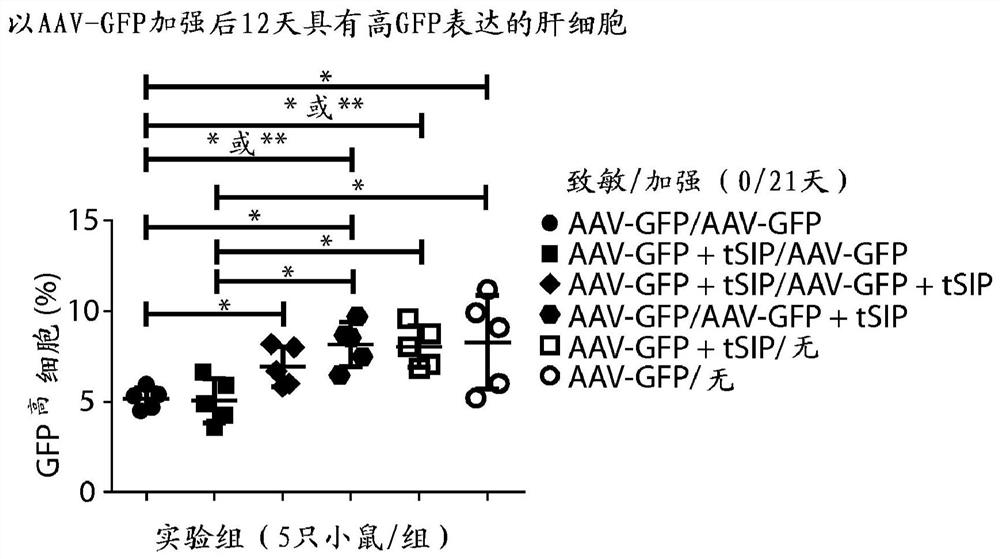
Methods and compositions for attenuating gene expression to modulate anti-viral transfer vector immune response
A transfer vector and gene expression technology, applied in the field of immunosuppressants, can solve complex problems and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0287] Example 1: Polymer Nanocarriers Containing Polymer-Rapamycin Conjugates (Prophetic)
[0288] Preparation of PLGA-rapamycin conjugate:
[0289] A PLGA polymer with acid end groups (7525 DLG1A, acid value 0.46 mmol / g, Lakeshore Biomaterials; 5 g, 2.3 mmol, 1.0 equiv) was dissolved in 30 mL of dichloromethane (DCM). Add N,N-dicyclohexylcarbodiimide (1.2 eq, 2.8 mmol, 0.57 g) followed by rapamycin (1.0 eq, 2.3 mmol, 2.1 g) and 4-dimethylaminopyridine (DMAP) (2.0 equiv, 4.6 mmol, 0.56 g). The mixture was stirred at room temperature for 2 days. The mixture was then filtered to remove insoluble dicyclohexylurea. The filtrate was concentrated to a volume of approximately 10 mL and added to 100 mL of isopropanol (IPA) to precipitate the PLGA-rapamycin conjugate. The IPA layer was removed and the polymer was washed with 50 mL IPA and 50 mL methyl tert-butyl ether (MTBE). The polymer was then dried under vacuum at 35°C for 2 days to give PLGA-rapamycin (about 6.5 g) as a wh...
Embodiment 2
[0294] Example 2: Preparation of gold nanocarriers (AuNC) containing rapamycin (prognostic)
[0295] Preparation of HS-PEG-rapamycin:
[0296] A solution of PEG acid disulfide (1.0 equiv), rapamycin (2.0-2.5 equiv), DCC (2.5 equiv) and DMAP (3.0 equiv) in anhydrous DMF was stirred overnight at room temperature. Insoluble dicyclohexylurea was removed by filtration, and the filtrate was added to isopropanol (IPA) to precipitate PEG-disulfide-di-rapamycin ester, washed with IPA and dried. The polymer was then treated with tris(2-carboxyethyl)phosphine hydrochloride in DMF to reduce the PEG disulfide to mercapto PEG rapamycin ester (HS-PEG-rapamycin). The resulting polymer was recovered by precipitation from IPA and dried as described previously and analyzed by H NMR and GPC.
[0297] Formation of gold NC (AuNC):
[0298] 500 mL of 1 mM HAuCl4 in water was heated to reflux for 10 min with vigorous stirring in a 1 L round bottom flask equipped with a condenser. Then 50 mL of ...
Embodiment 3
[0301] Example 3: Mesoporous Silica Nanoparticles with Attached Ibuprofen (Prophetic)
[0302] The mesoporous SiO2 nanoparticle cores were produced by a sol-gel method. Cetyltrimethylammonium bromide (CTAB) (0.5 g) was dissolved in deionized water (500 mL), then 2M aqueous NaOH (3.5 mL) was added to the CTAB solution. The solution was stirred for 30 minutes, then tetraethoxysilane (TEOS) (2.5 mL) was added to the solution. The resulting gel was stirred at a temperature of 80° C. for 3 hours. The white precipitate formed was captured by filtration, washed with deionized water and dried at room temperature. The remaining surfactant was then extracted from the particles by suspending overnight in HCl in ethanol. The particles were washed with ethanol, centrifuged, and redispersed under sonication. This washing process was repeated two more times.
[0303] The SiO2 nanoparticles were then functionalized with amino groups using (3-aminopropyl)-triethoxysilane (APTMS). For t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com