Application of an ionizable lipid compound in nucleic acid drug delivery system
A nucleic acid drug and delivery system technology, applied in drug delivery, nano-drugs, medical preparations of non-active ingredients, etc., can solve the problems of increased drug dose, increased liver metabolic burden, and low delivery efficiency of the delivery system, reducing the The effect of cumulative side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Synthetic route of compound 1:
[0093]
[0094] Step 1: Synthesis of Compound 1-1:
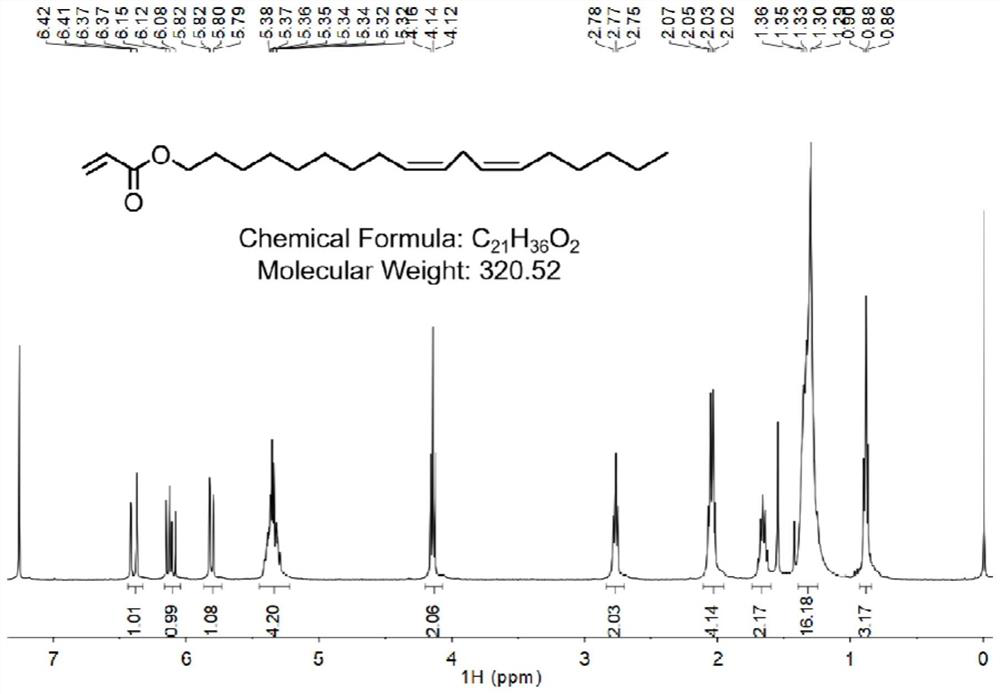
[0095] Add linolenic alcohol (0.267 g, 1 mmol) and triethylamine (0.133 g, 1.3 mmol) to the ice-water bath in the reaction flask, add dichloromethane (6 mL), and dissolve acryloyl chloride (0.11 g, 1.2 mmol) in dichloromethane (2.2 mL), slowly dropped into the reaction flask, the reaction continued for 10 minutes, the reaction was kept below 10° C., and finally the ice bath was removed, and the reaction solution was reacted at room temperature for 2 hours. Washed with saturated brine to obtain crude product, which was purified by chromatography (silica gel column, eluent is petroleum ether containing 0.5% EA (volume percent)), and the pure product was evaporated to obtain compound 1 as light yellow oil -1(2-Allenic acid (9Z, 12Z)-octadecadienyl ester) (0.173 g, yield: 50%) The hydrogen spectrum of compound 1-1 is shown in figure 1 .
[0096] 1H NMR (400MHz, CDCl 3 )δ: 6.41 (dd, ...
Embodiment 2
[0101] Synthetic route of compound 2
[0102]
[0103] Step 1: Synthesis of Compound 2-1
[0104]6-Bromohexanoic acid (1.0 g, 5.13 mmol) and undecanol (1.77 g, 10.25 mmol) were dissolved in dichloromethane (60 mL) and 1-(3-dimethylaminopropyl)-3-ethyl was added carbodiimide hydrochloride (EDC hydrochloride, 0.98 g, 5.13 mmol) and DMAP (0.125 g, 1.03 mmol). The mixture was stirred at normal temperature for 18 hours. After the reaction was over, it was diluted with DCM (200 mL) and saturated with NaHCO 3 (100 mL) and brine (100 mL). Combine the organic layers with anhydrous Na 2 SO 4 Drying and removal of solvent in vacuo gave crude product, which was purified by chromatography (silica gel column, eluent: petroleum ether containing 0.5% EA (v / v)) and evaporated to give compound 2 as a pale yellow oil -1(undecyl 6-bromohexanoate) (0.69 g, 38.6% yield). For the hydrogen spectrum of compound 2-1, see Figure 5 .
[0105] 1H NMR (400MHz, CDCl 3 )δ: 4.10(t, J=6.6Hz, 2H),...
Embodiment 3
[0110] Synthetic route of compound 3
[0111]
[0112] Step 1: Synthesis of Compound 3-1
[0113] 8-Bromooctanoic acid (1.139 g, 5.13 mmol) and 3,7-dimethyloct-6-en-1-ol (citronellol, 1.599 g, 10.25 mmol) were dissolved in dichloromethane (60 mL), fully After dissolution, EDC hydrochloride (0.98 g, 5.13 mmol) and DMAP (0.125 g, 1.03 mmol) were added. The mixture was stirred at normal temperature for 18 hours. After the reaction was over, it was diluted with DCM (200 mL) and saturated with NaHCO 3 (100 mL) and brine (100 mL). Combine the organic layers with anhydrous Na 2 SO 4 Dry, remove the solvent in vacuo to give the crude product, which is purified by chromatography (silica gel column, eluent is petroleum ether containing 0.5% EA (v / v)) and evaporated to give compound 3 as a pale yellow oil -1(3,7-dimethyloct-6-enyl 6-bromohexanoate) (0.648g, 35%) See the hydrogen spectrum of compound 3-1 Figure 8 .
[0114] 1H NMR (400MHz, CDCl 3 )δ: 5.09(s, 1H), 4.18-4.01(m,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com