Method for producing aminomethyl aromate
A technology of aminomethyl aromatics and manufacturing methods, which is applied to the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of large loads and achieve the effects of inhibiting deterioration and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] (manufacture of p-xylylenediamine)
[0111] The methanol slurry (catalyst amount: 5.90 g) of the catalyst was charged into a 500 mL autoclave container, and adjusted so that the total mass of methanol became 39.5 g. Then, 51.8 g of terephthalonitrile, 197.3 g of m-xylene, and 1.52 g of a 25% tetramethylammonium hydroxide aqueous solution (4.2 mmol, 0.38 g as tetramethylammonium hydroxide) were charged.
[0112] Nitrogen replacement was performed by pressurizing the inside of the reactor to 0.5 MPa with nitrogen and returning to atmospheric pressure. This nitrogen substitution was performed a total of 3 times, and then using hydrogen, a total of 3 hydrogen substitutions were performed in the same manner.
[0113] The hydrogen pressure was set at 8.0 MPa, the temperature was raised to 100° C. while stirring at 1200 rpm, and the reaction was carried out at 8.0 MPa and 100° C. while supplying hydrogen. The reaction was terminated at the point when the hydrogen was consume...
Embodiment 2
[0115]
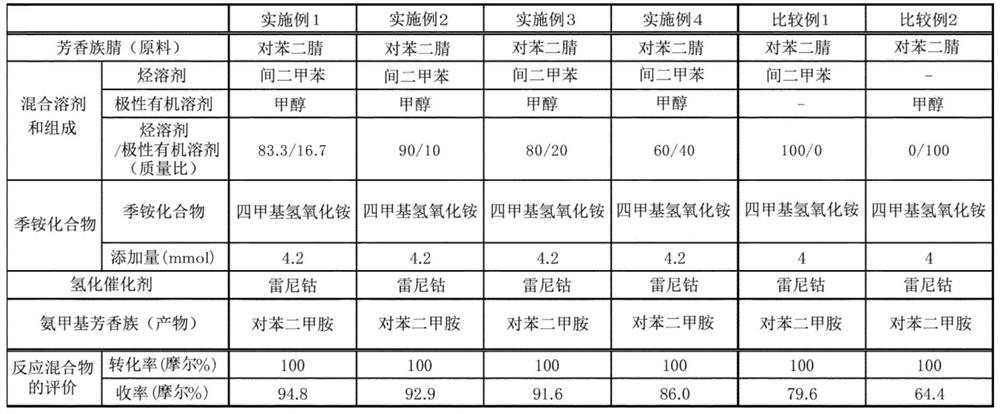
[0116] The total amount of methanol and m-xylene is the same as in Example 1, and the mass ratio of methanol and m-xylene is adjusted so that it becomes the ratio of Table 1, and the same operation as in Example 1 is performed to perform the reaction. . Table 1 shows the conversion rate of terephthalonitrile and the yield of p-xylylenediamine.
[0117]
[0118] The amount of m-xylene was 236.8 g, and the reaction was performed in the same manner as in Example 1 except that methanol was not used. In addition, as a catalyst, the methanol slurry was replaced with the m-xylene slurry. Table 1 shows the conversion rate of terephthalonitrile and the yield of p-xylylenediamine.
Embodiment 5
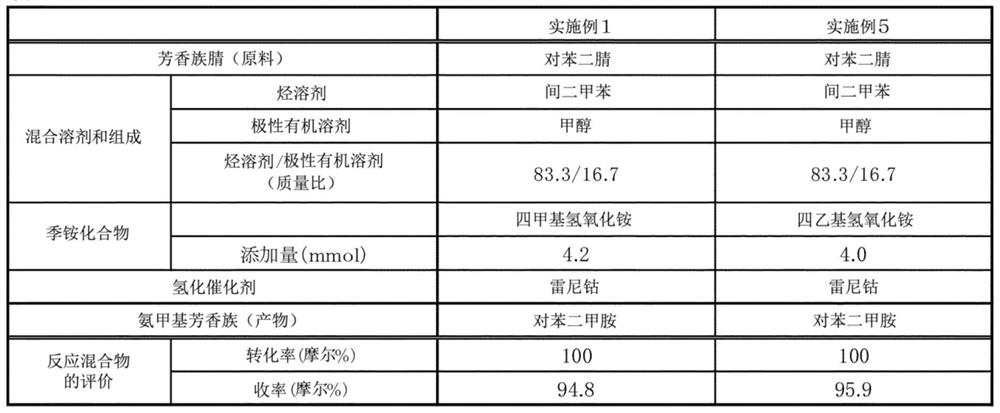
[0138] A reaction was performed by performing the same operation as in Example 1, using a tetraethylammonium aqueous solution instead of the tetramethylammonium hydroxide aqueous solution in Example 1. Table 3 shows the conversion rate of terephthalonitrile and the yield of p-xylylenediamine.
[0139] [table 3]
[0140] table 3
[0141]
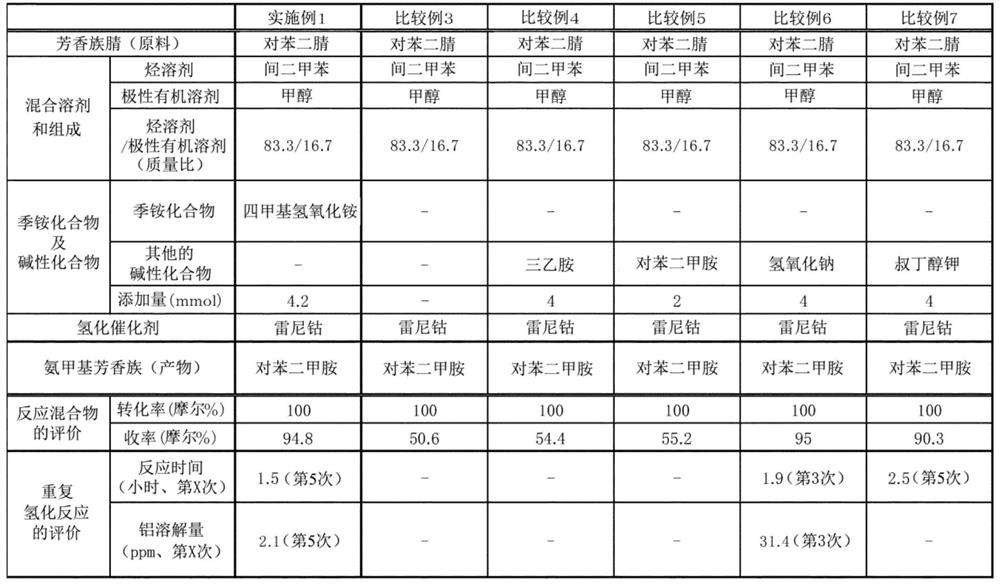
[0142] From the results of Tables 1 to 3, it can be seen that p-xylylenediamine can be obtained in a high yield when using the production method of the example, and furthermore, even if the reaction is repeated, the catalyst can be reacted in a short time without deterioration. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com