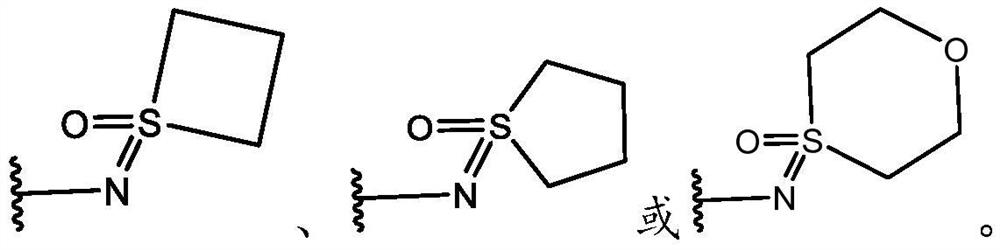
KIF18A inhibitors
A pharmaceutical, alkyl technology, applied in the field of managing cell proliferation and treating cancer, preparing compounds of formula I, regulating KIF18A compounds and compositions, and KIF18A inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0340] Preparation of synthetic intermediates
[0341] Preparation of ring Ar 1 intermediate
[0342] Intermediate 1: 3-(4,4-Difluoropiperidin-1-yl)-5-methylaniline
[0343]
[0344] step 1 : To a solution of 1-bromo-3-methyl-5-nitrobenzene (10 g, 46.3 mmol) and 4,4-difluoropiperidine hydrochloride (10.9 g, 69.4 mmol) in toluene (50 mL) was added Sodium tert-butoxide (13.3g, 139mmol), Pd 2 (dba) 3 (4.24g, 4.63mmol) and xantphos (2.68g, 4.63mmol). The reaction mixture was heated at 100 °C for 1.5 h and allowed to cool to room temperature. The reaction mixture was diluted with water, after bed and washed with EtOAc. The organic extract was washed with brine, washed with Na 2 SO 4 Dry, filter, and concentrate in vacuo. The crude product was purified by flash column chromatography (eluting with 10% to 20% EtOAc in petroleum ether) to afford 4,4-difluoro-1-(3-methyl-5-nitro as a gray solid Phenyl)piperidine (2.3 g, 9.0 mmol, 19% yield). 1 H NMR (400MHz, DMSO...
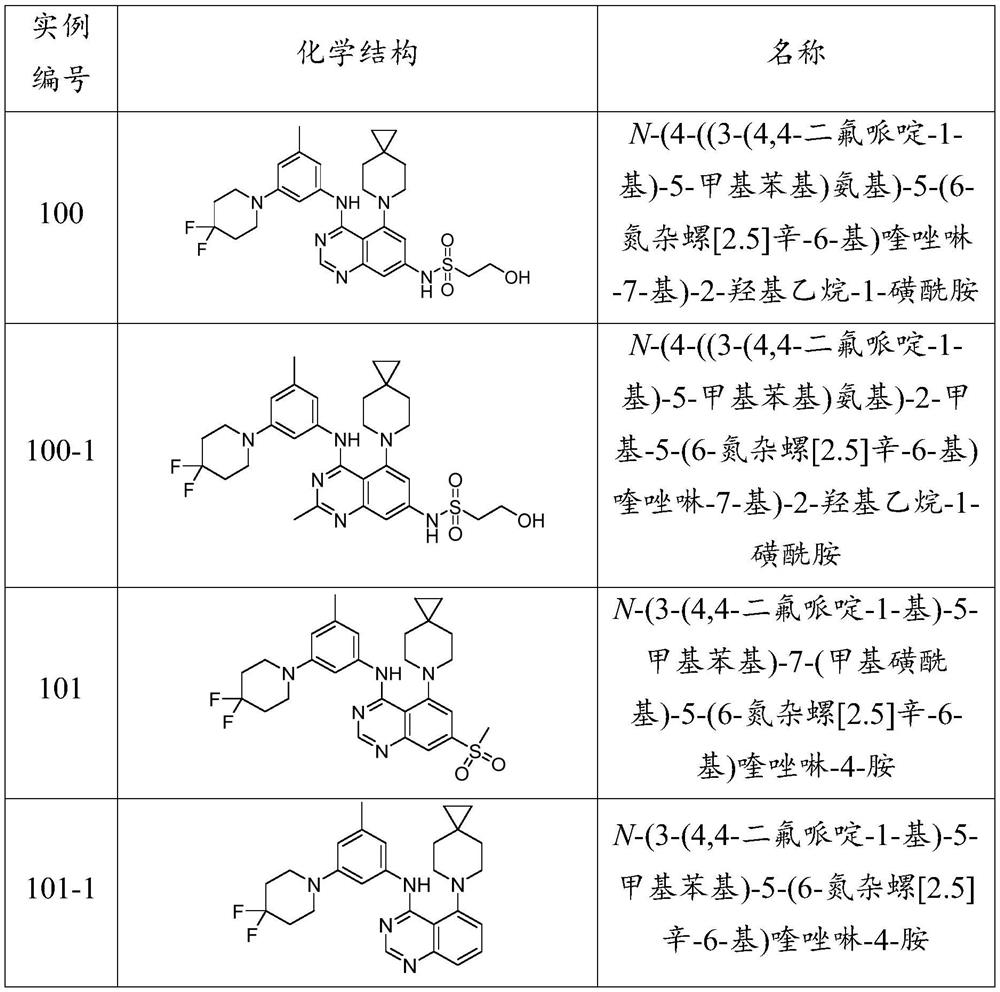
example 100
[0393] Example 100: N-(4-((3-(4,4-difluoropiperidin-1-yl)-5-methylphenyl)amino)-5-(6-azaspiro [2.5] Oct-6-yl)quinazolin-7-yl)-2-hydroxyethane-1-sulfonamide
[0394]
[0395] At rt, to 7-bromo-N-(3-(4,4-difluoropiperidin-1-yl)-5-methylphenyl)-5-(6-azaspiro[2.5]octyl- To a solution of 6-yl)quinazolin-4-amine (0.125 g, 0.230 mmol, intermediate 12) and 2-hydroxyethane-1-sulfonamide (0.058 g, 0.461 mmol) in DMF (2 mL) was added Tripotassium phosphate (0.147g, 0.691mmol), copper(I) iodide (0.088g, 0.461mmol) and (1R,2R)-N,N'-dimethyl-1,2-cyclohexanediamine (0.033 g, 0.230mmol) and heated at 95°C for 16h. Pass the reaction mixture through Pad filtered and washed with EtOAc. The combined organic extracts were washed with brine, washed with Na 2 SO 4 Drying, filtration and evaporation gave crude compound. The crude product was passed through preparative HPLC [Sun fireC-18 (150x19) mm, 5.0 μm, CH 3 CN / H 2 0.1% TFA in O] purified to give N-(4-((3-(4,4-difluoropiperidin-1-...
preparation example 100
[0397]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com