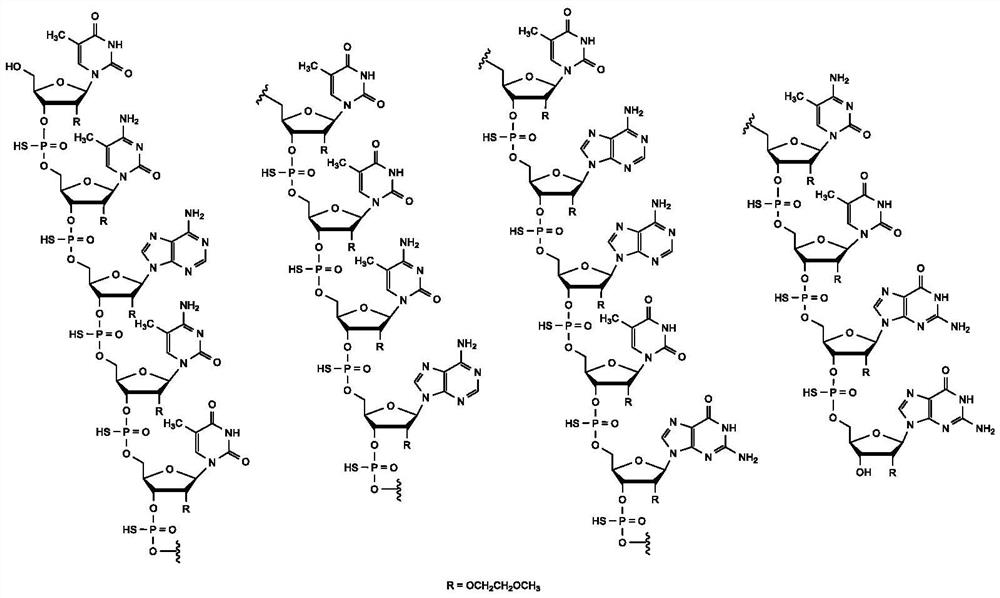
Methods of treating or preventing spinal muscular atrophy
A spinal muscular atrophy, antisense compound technology, applied in gene therapy, biochemical equipment and methods, pharmaceutical formulations, etc., can solve the problems of lack of exon 7 truncated form, inactivity, and unstable form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: PK / PD analysis of Nuxinargen population
[0128] In a Nosinerzen clinical study of subjects with SMA, subjects treated before or shortly after the onset of symptoms and diagnosis of SMA compared to subjects with a longer duration of disease at the start of treatment Usually has better results. In the infantile-onset SMA study, most deaths among infants treated with norcinergen occurred within the first 2 months of the study, before completion of the loading dose regimen and achievement of steady-state norcinergen concentrations. Given the rapid decline associated with Type I SMA and the importance of early treatment, faster attainment of higher norcinergen concentrations may further enhance the efficacy of norcinergen and prevent or further ameliorate disease progression in patients with SMA. Results from the studies of infantile-onset SMA as well as those of sham-controlled studies in subjects with late-onset SMA support the conferring of greater benefit ...
Embodiment 2
[0129] Example 2: Exposure-response relationships
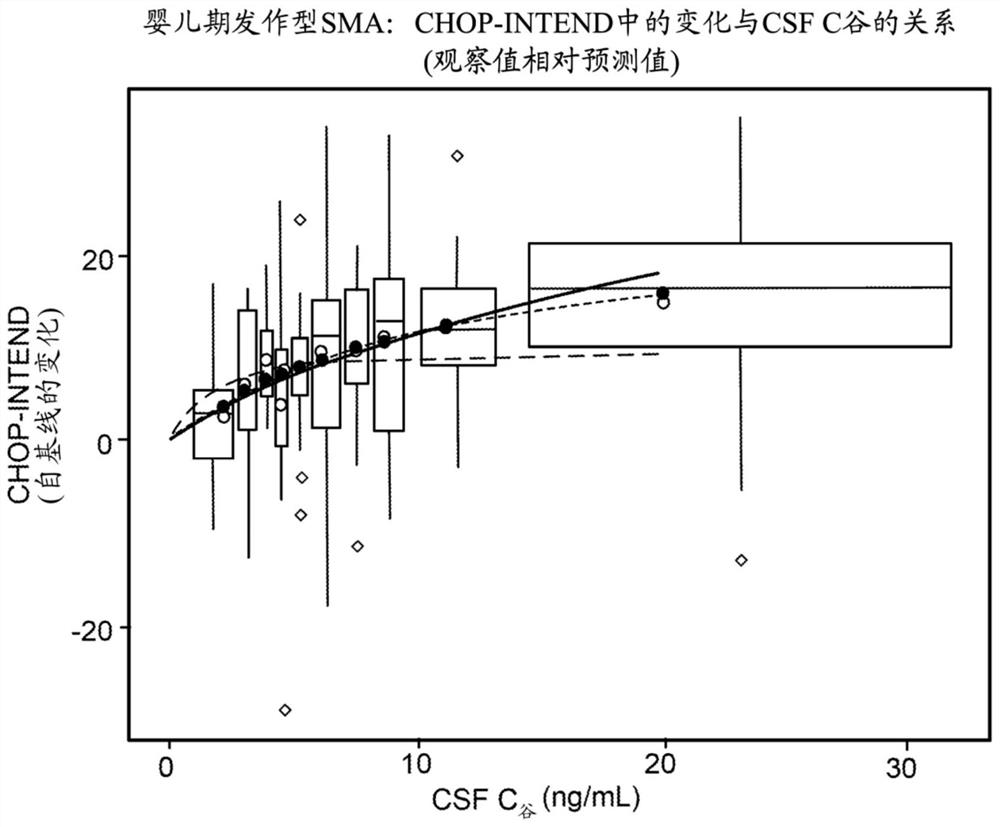
[0130] An exploratory exposure-response analysis was performed using data from 14 subjects with infancy-onset SMA who received norcinergen during the Nocinergen clinical development program. The results of the analysis demonstrated a statistically significant positive correlation between Nosinagen CSF exposure and motor function. A dose-related trend was observed in subjects achieving exercise milestones, with subjects receiving a 12 mg loading dose gaining Improvement earlier and to a greater extent. All subjects received a maintenance dose of 12 mg every 4 months after day 85. The response curves for the 2 groups started to separate around day 29 and became wider as the study progressed.
[0131] Greater improvements in motor function were also observed in subjects with infantile-onset SMA who received more frequent dosing (on days 1, 15, 29, and 12 mg loading dose on day 64 and 12 mg maintenance dose every 4 months)....
Embodiment 3
[0135] Example 3: Loading dose optimization
[0136] PK simulations were performed with the goal of identifying loading regimens that more rapidly produced targeted higher drug exposures (approximately 2-fold increase) at lower intrathecal (IT) doses. These simulations were performed for both the infantile-onset SMA population and the late-onset SMA population after 2 years of treatment, and were based on patients with infantile-onset, late-onset, or presymptomatic Population PK Models Generated from SMA Patient Data. Maintenance dosing frequency was not evaluated as previous modeling indicated that a dosing frequency of 4 months optimally maintained achieved CSF concentrations at steady state.
[0137] Use 20ng / mL as clinical CSF C 谷 Target concentrations and using the predicted CSF PK profile from 24 mg norcinargen (4 loading doses followed by a maintenance dose every 4 months) as a reference dosing regimen, simulations were performed to evaluate the efficacy of using hi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap