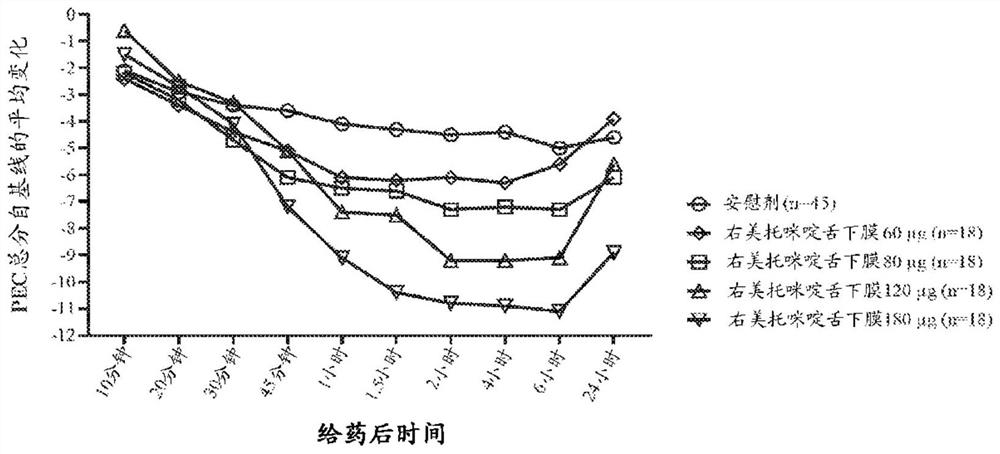
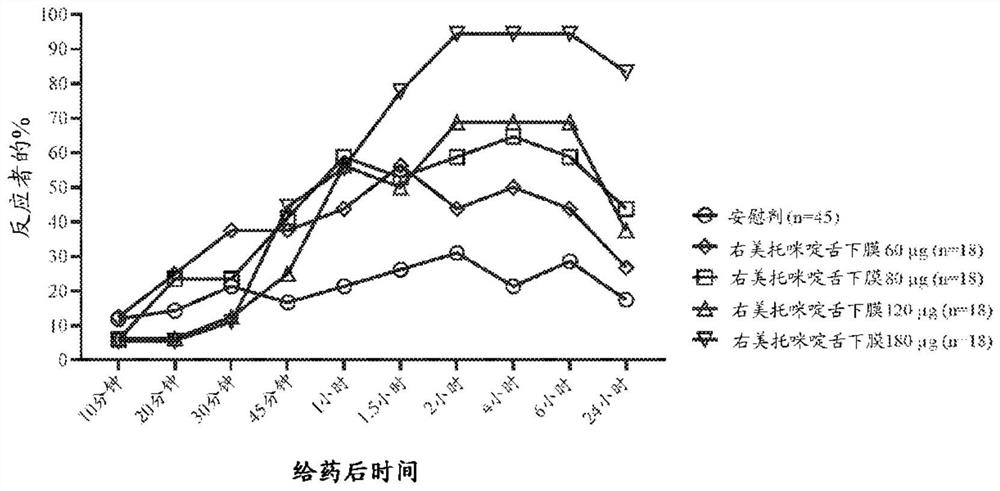
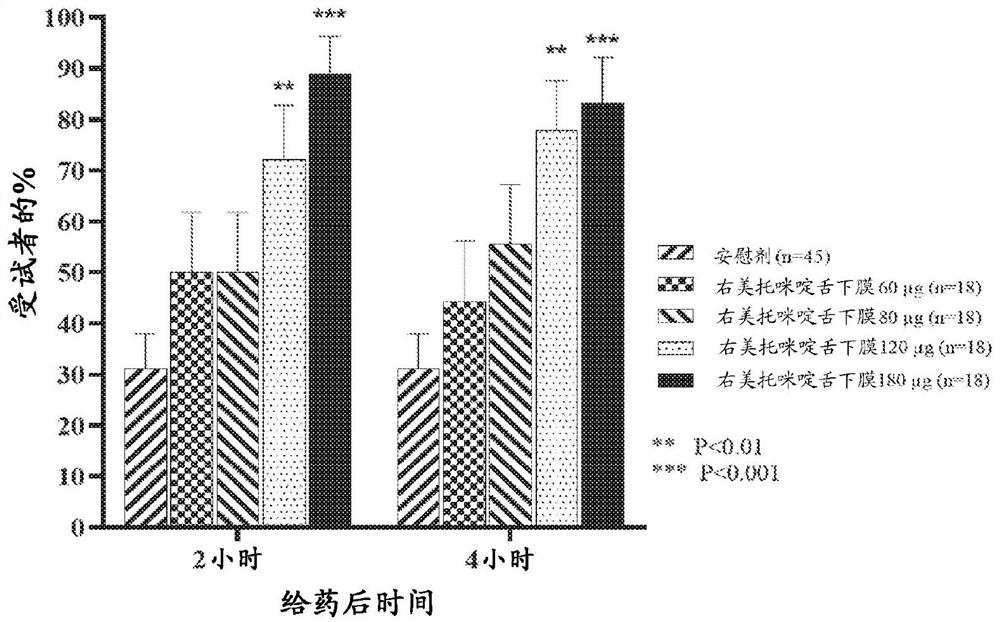
Non-sedative dexmedetomidine treatment regimen
A dexmedetomidine and sedation technology, applied in the field of non-sedative dexmedetomidine treatment plan, can solve problems such as unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0622] Embodiment 1. A method of treating agitation or symptoms of agitation in a human subject with schizophrenia or bipolar disorder without additionally inducing significant sedation, the method comprising producing as by TO Dexmedetomidine or a pharmaceutically acceptable salt thereof was administered at a dose to a mean total exposure of dexmedetomidine of about 3800 ng*h / L as measured by plasma AUC of T∞.
Embodiment approach 2
[0623] Embodiment 2. A method of treating agitation or symptoms of agitation in a human subject with schizophrenia or bipolar disorder without additionally inducing significant sedation, the method comprising producing as by TO Dexmedetomidine or a pharmaceutically acceptable salt thereof is administered at a dose to a total exposure of dexmedetomidine of about 600 to about 12600 ng*h / L as measured by plasma AUC of T∞.
Embodiment approach 3
[0624] Embodiment 3. A method of treating agitation or symptoms of agitation in a human subject with schizophrenia or bipolar disorder without additionally inducing significant sedation, the method comprising producing as by TO Dexmedetomidine or a pharmaceutically acceptable salt thereof was administered at a dose to a mean total exposure of dexmedetomidine of about 1800 ng*h / L as measured by plasma AUC of T∞.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com