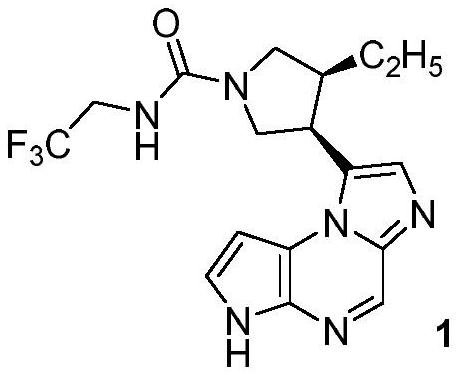
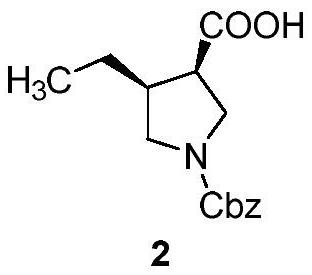
Preparation method of chiral 4-alkyl-pyrrole-3-formic acid compound
A compound, a technology of benzyloxycarbonylpyrrolidine, which is applied in the field of preparation of 4-alkyl-pyrrole-3-carboxylic acid compounds, can solve problems such as expensive transition metal catalysts, and achieve the effects of reducing production costs and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
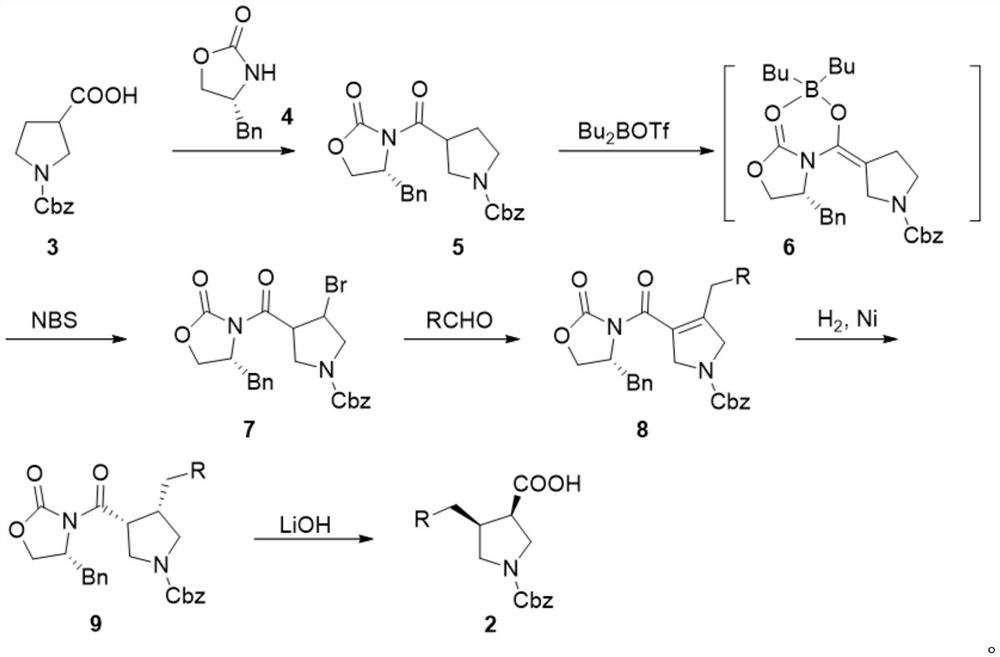
[0051] Example 1 Synthesis of Compound 5.
[0052]
[0053] Compound 4 (100g, 0.56mol, 1.0eq.) was dissolved in tetrahydrofuran (500ml), cooled to below -40°C, and 1mol / L tetrahydrofuran solution of lithium bistrimethylsilylamide (677ml, 0.677mol, 1.2 eq.) is added dropwise, and the rate of addition is controlled so that the temperature of the reaction system is not higher than -40°C. After the dropwise addition is completed, stir for 10 minutes, and dissolve compound 3 (154.7g, 0.62mol, 1.1eq.) in tetrahydrofuran (500ml ), drop it into, control the rate of addition, and keep the temperature not higher than -40°C. After completion of the dropwise addition, react at -50 to -40°C for 1 hour, add saturated ammonium chloride solution (550ml), add ethyl acetate (500ml), leave to stand for layers, and use saturated sodium chloride solution (300ml× 3) Wash, concentrate the organic phase to dryness under reduced pressure below 45°C, add ethyl acetate (320ml) to the residue, stir t...
Embodiment 2
[0054] Synthesis of Example 2 Compound 5.
[0055] Compound 4 (100g, 0.56mol, 1.0eq.) was dissolved in 2-methyltetrahydrofuran (500ml), cooled to below -40°C, and 1mol / L tetrahydrofuran solution of lithium bistrimethylsilylamide (564ml, 0.564mol, 1.0eq.) was added dropwise, and the rate of addition was controlled so that the temperature of the reaction system was not higher than -40°C. After the dropwise addition was completed, stir for 10 minutes to dissolve compound 3 (154.7g, 0.62mol, 1.1eq.) Add dropwise into 2-methyltetrahydrofuran (500ml), control the rate of addition, and keep the temperature not higher than -40°C. After completion of the dropwise addition, react at -50 to -40°C for 1 hour, add saturated ammonium chloride solution (450ml), add ethyl acetate (500ml), let stand to separate layers, and use saturated sodium chloride solution (300ml× 3) Wash, concentrate the organic phase to dryness under reduced pressure below 45°C, add ethyl acetate (320ml) to the residue...
Embodiment 3
[0056] Example 3 Synthesis of Compound 5.
[0057] Compound 4 (100g, 0.56mol, 1.0eq.) was dissolved in tetrahydrofuran (500ml), cooled to below -40°C, and 1mol / L tetrahydrofuran solution of lithium bistrimethylsilylamide (790ml, 0.790mol, 1.4 eq.) is added dropwise, and the rate of addition is controlled so that the temperature of the reaction system is not higher than -40°C. After the dropwise addition is completed, stir for 10 minutes, and dissolve compound 3 (140.7g, 0.56mol, 1.0eq.) in tetrahydrofuran (500ml ), drop it into, control the rate of addition, and keep the temperature not higher than -40°C. After completion of the dropwise addition, react at -50 to -40°C for 1 hour, add saturated ammonium chloride solution (640ml), add ethyl acetate (500ml), leave to stand for layers, and use saturated sodium chloride solution (300ml× 3) Wash, concentrate the organic phase to dryness under reduced pressure below 45°C, add ethyl acetate (320ml) to the residue, stir to dissolve, ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap