Methods of treatment using genetically modified autologous T cell immunotherapy
A technology of cells, cell populations, applied in the therapeutic area of immunotherapy using genetically modified autologous T cells that can address the high unmet need for clinical benefit to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0279] Example 1 Neotcr product
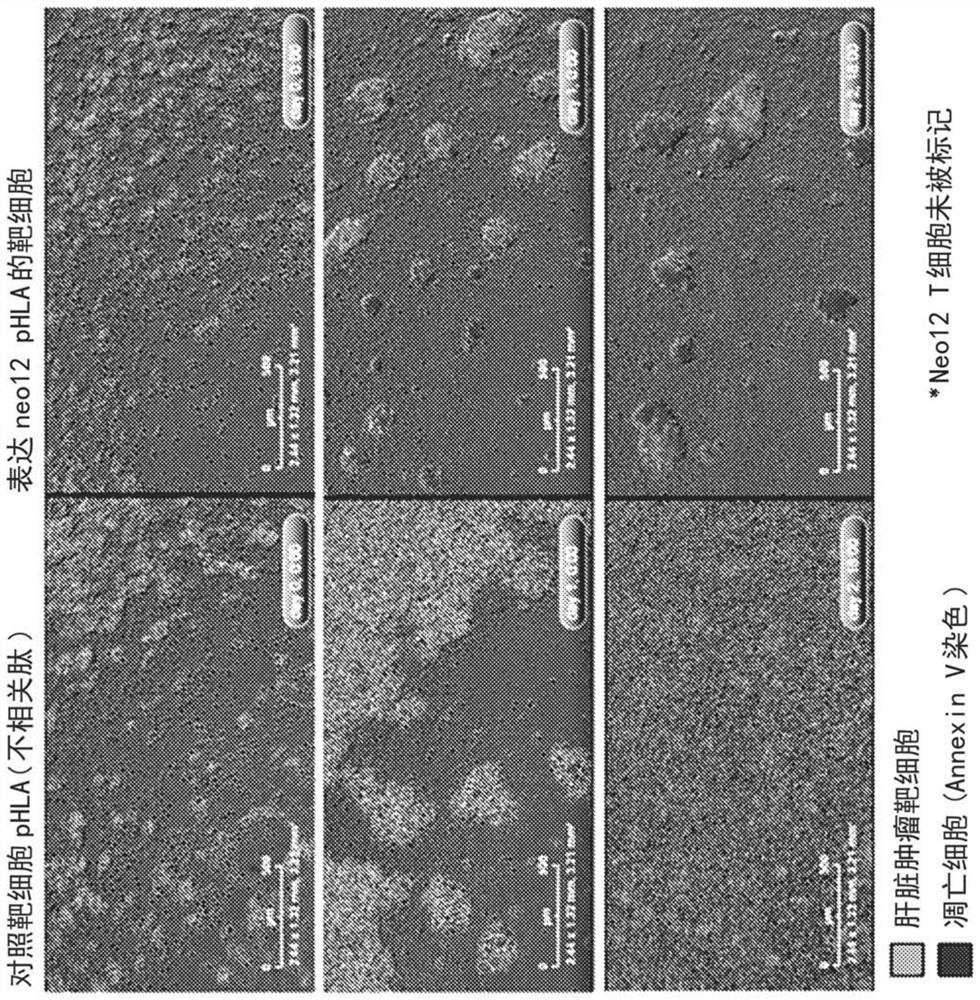
[0280] Antigen -specific target T cell kill. The treatment of mutant -targeted, personalized, over -success T cell receptors (NeoTCR products) is an immunotherapy mode, which aims to release the ability of specific identification and killing the cells of the immune system. NeoTCR cells are transformed to express Neo12 TCR (a representative new table), which is produced by NEO12 specific CD8 T cells separated from the blood of patients with melanoma, and tumor cells that are homologous or non -matching tumor cells with homologous or non -matching tumor cells Cultivate a few days to collect delay live micro -surgical images. Use delay live body micro -ingrains (0, 1 and 2 days; see figure 1 ) The representative image obtained shows that NeoTCR-T cells trained by target T cells that express the same-origin NEO12 peptide-HLA have strong antigen-specific cytotoxic activity and proliferation capabilities (right column) Target T cells do not have antigen...
Embodiment 2
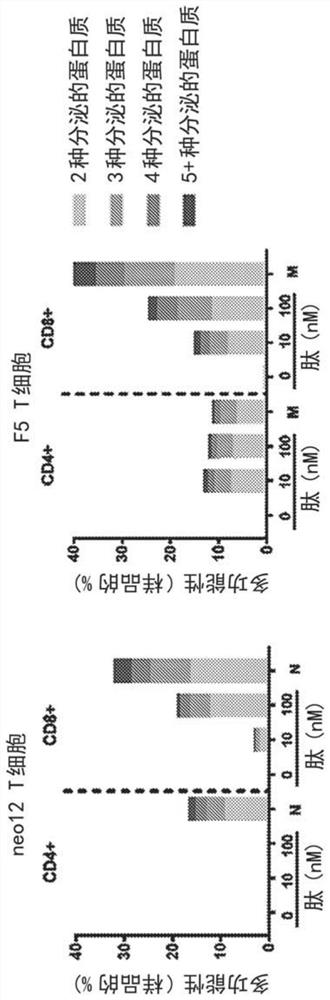
[0291] Example 2 and multi -TCR Neotcr products
[0292] In certain embodiments, each Neotcr product contains a single NeoTCR, which is transformed by a precision genome to CD4+and CD8+T cells. In view of the design of NeoTCR products, NeoTCR with short -term mutation, NEOTCR products with only one (1) NeoTCR may be sufficient and effective in treating proliferative diseases (such as cancer).
[0293] The number of NeoTCR in Neotcr products does not perform the limit. Instead, the number of NeoTCR included in NeoTCR products is based on the following number of Neotcr: 1) the number of NeoTCR based on patient tumors and blood samples, or 2) hopes to have multiple NeoTCR in given products.
[0294] In some embodiments, Neotcr products include a single Neotcr. NeoTCR products include single (1) Neotcr, which is made by screening the neotcr in the patient's tumor and blood samples and selected a single (1) species Neotcr to be transformed into NEOTCR products to manufacture. In some e...
Embodiment 3
[0316] Example 3 Neotcr product combination therapy
[0317] Overview.
[0318] NeoTCR products can be used alone or for combined treatment. The combination therapy may include applying NeotCR products and applying 1, 2, 3, 4, 5, 6, or 7 or more other treatment agents. In some embodiments, the combination therapy includes the application of Neotcr products and at least one other treatment agent. In some embodiments, the combination therapy includes the application of NeoTCR products and at least two other treatment agents. In some embodiments, the combination therapy includes the application of Neotcr products and at least three other therapeutic agents. In some embodiments, the combination therapy includes the application of Neotcr products and at least four other treatment agents. In some embodiments, the combination therapy includes the application of NeoTCR products and at least five other treatment agents. In some embodiments, the combination therapy includes the application...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com