Anti-wt1/hla bi-specific antibody
A specific and antibody-based technology, applied in the field of antibodies against cytosolic proteins, can solve problems such as low TCR affinity, lack of persistence of effector cells, and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
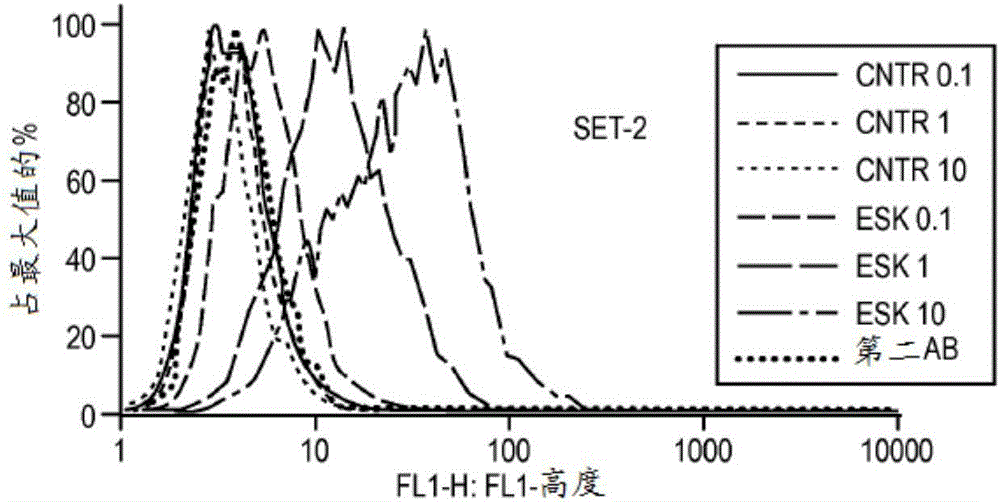
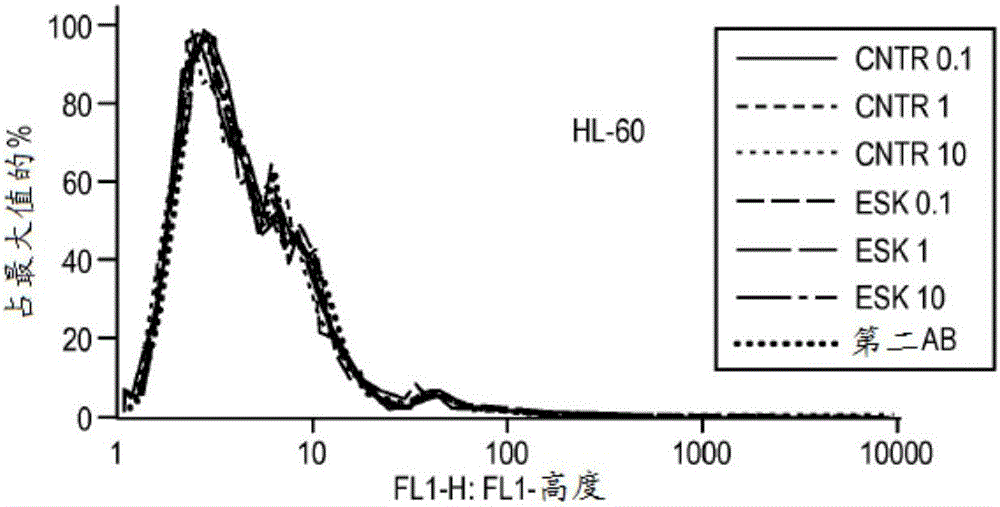
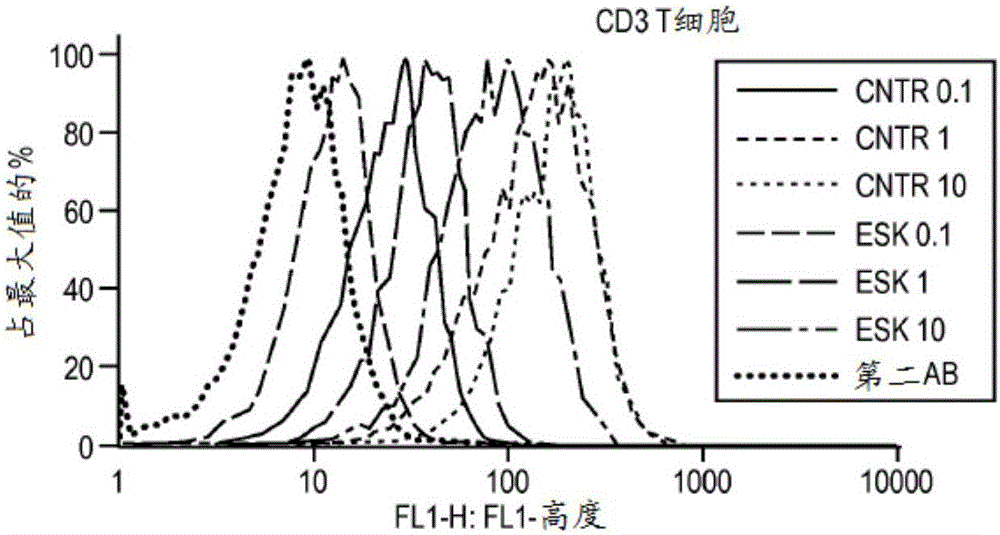
[0172] Materials and methods
[0173] Cell samples, cell lines and antibodies. Peripheral blood mononuclear cells (PBMC) from HLA-typed healthy donors and patients were obtained by Ficoll density centrifugation. The sources used to obtain human leukemia and solid tumor cell lines have been described previously (Dao et al., supra). The cell lines used in this study include: AML lines HL60, SET-2, Ph+ALL line BV173, mesothelioma cell lines JMN and MSTO. All cells have been HLA-typed. at 37C / 5% CO 2 The cell lines were cultured in RPMI 1640 supplemented with 5% FCS, penicillin, streptomycin, 2mmol / L glutamine and 2-mercaptoethanol. Tumor cells for animal studies were transduced with GFP / luciferase as previously described (Dao et al., supra). ESK1 and its control human IgG1 were produced by Eureka Therapeutics Inc (Emeryville, CA), and APC conjugation was performed according to the manufacturer's instructions (Dao et al., supra). Monoclonal antibody (mAb) against human HLA-A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com