Application of Hnf-1alpha gene modified mesenchymal stem cells in prevention and treatment of liver cancer
A kind of high-quality stem cell technology, which is applied in the field of stem cell therapy to prevent the recurrence and metastasis of liver cancer in the early stage, and can solve the problems of damage and secondary trauma of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
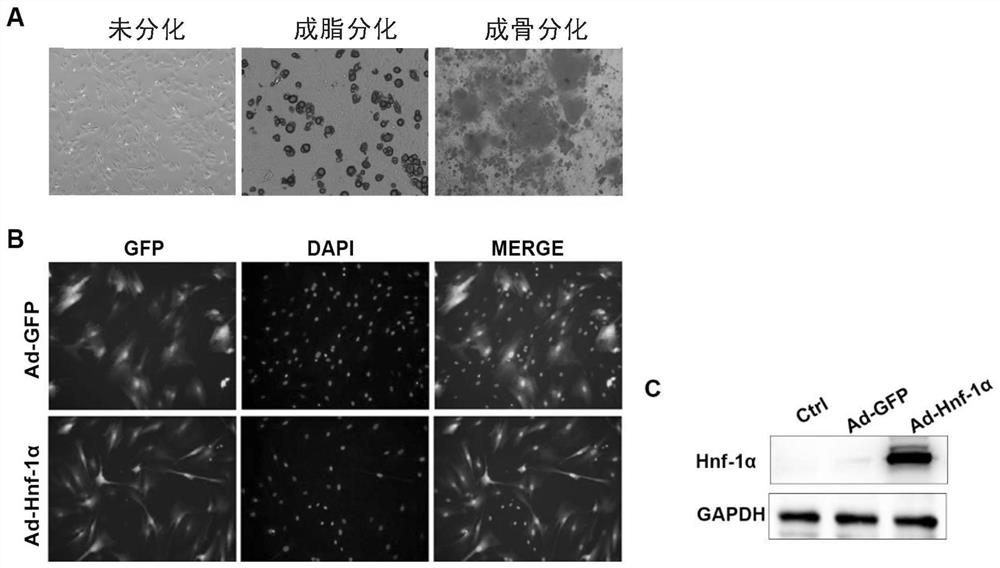
[0089] Example 1. Isolation and identification of rat bone marrow primary mesenchymal stem cells and preparation of Hnf-1α overexpressing mesenchymal stem cells
[0090] In this example, rat bone marrow-derived mesenchymal stem cells (rBMSCs) were used as an example to study.
[0091] (1) Isolation and in vitro culture of rat bone marrow mesenchymal stem cells
[0092] Bone marrow MSCs were isolated and cultured in vitro from SD rats by adherent culture method. Male SD rats 200-250 g were taken, anesthetized with 10% chloral hydrate, and the femur was removed and placed in L-DMEM medium after routine disinfection. In the ultra-clean bench, rinse the bone marrow cavity with L-DMEM medium, collect the bone marrow suspension, centrifuge for 5 minutes (1500 rpm), discard the supernatant, collect the cell pellet, add L-DMEM+10% fetal bovine serum+ 100U / mL penicillin + 100U / mL streptomycin, counted by hemocytometer, 1×10 5 / cm 2 Inoculated in a 10cm petri dish, in L-DMEM+10% fet...
Embodiment 2
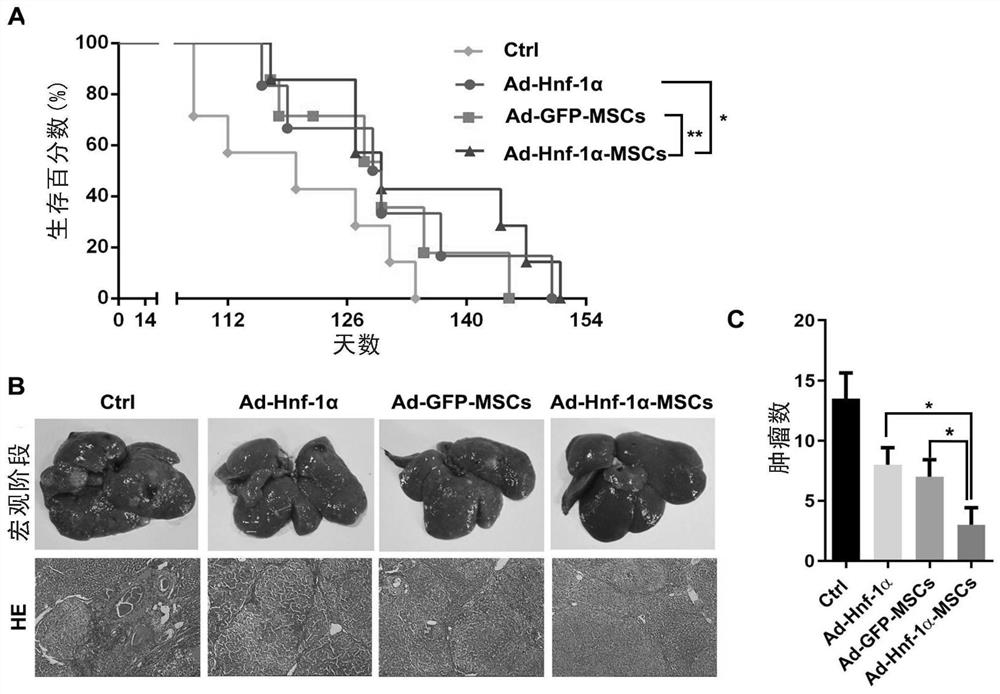
[0108] Example 2. Study on the effect of Hnf-1α gene-modified MSCs on the occurrence and development of DEN-induced primary liver cancer in rats
[0109] After adaptive feeding for one week, 20 SD rats were randomly divided into 4 groups with 5 rats in each group, namely PBS infusion group (Ctrl), Ad-GFP-MSCs infusion group (Ad-GFP-MSCs), Ad-Hnf -1α-MSCs infusion group (Ad-Hnf-1α-MSCs) and Ad-Hnf-1α infusion group, experimental animals in all treatment groups were fed DEN water (0.1% diethylnitrosamine), and animals in each experimental group were fed with DEN water (0.1% diethylnitrosamine). Tail vein injection started from the 4th week of DEN induction (early stage of cancer induction, or called mutation stage), 1.5×10 6 cells / only / 800μL; the control group (Ctrl) was infused with 800μL of PBS; the tail vein was injected once every two weeks, three times in total. Then continue to feed DEN water until the end of 14 weeks, and change to ordinary drinking water. The survival ...
Embodiment 3
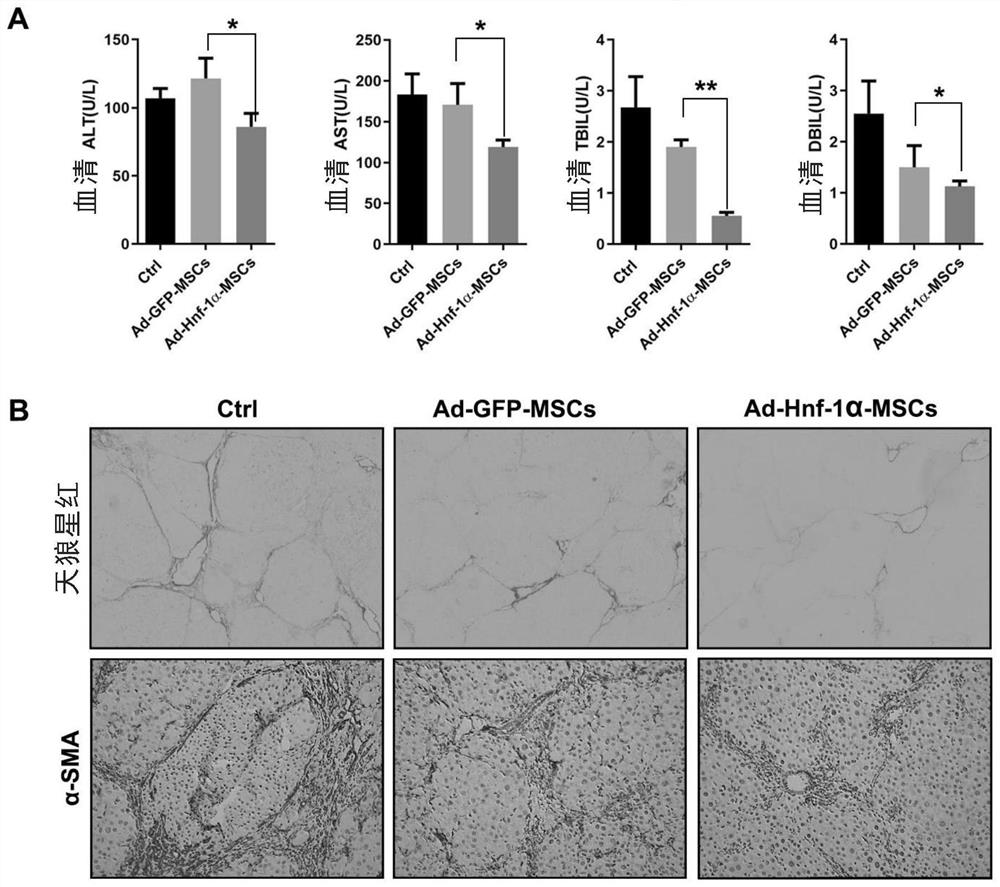
[0112] Example 3. Effect of Hnf-1α gene-modified mesenchymal stem cells on DEN-induced liver injury repair
[0113] After 15 SD rats were adaptively fed for one week, they were randomly divided into 3 groups with 5 rats in each group, namely PBS infusion group (Ctrl), Ad-GFP-MSCs infusion group (Ad-GFP-MSCs) and Ad-Hnf In the -1α-MSCs infusion group (Ad-Hnf-1α-MSCs), all the experimental animals in the treatment groups were fed DEN water (0.1% diethylnitrosamine). Tail vein injection, 1.5 x 10 6 cells / only / 800μL; the control group was infused with 800μL of PBS; the tail vein was injected once every two weeks, three times in total. At the 8th week of DEN treatment, the experimental animals were sacrificed, and the animal serum and liver tissue were collected for relevant detection.
[0114] The results of biochemical analysis showed that compared with the Ctrl control group, the levels of liver function indexes such as ALT, AST, TBIL and DBIL in the serum of the animals in th...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap