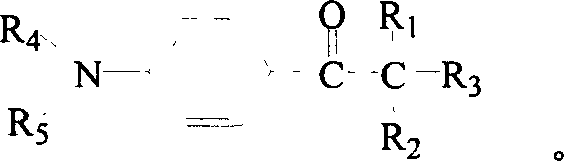Photosensitive quaternary ammonium salt and its preparation method and use
A technology of quaternary ammonium salt and quaternary ammonium salt compound is applied in the field of double-effect photosensitive quaternary ammonium salt, which can solve the problems of low photoamine production efficiency, unattempted dual-curing performance, and difficulty in obtaining raw materials, and achieves the effect of adjusting comprehensive performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
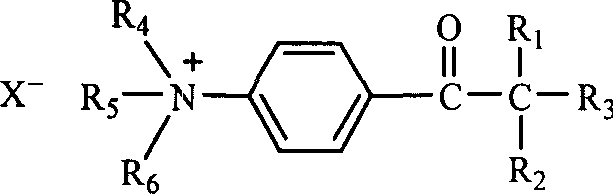
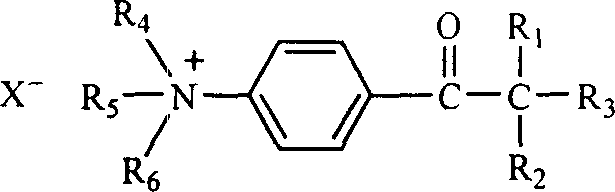
[0031] Preparation of photosensitive quaternary ammonium salt: add 0.04mol 2-benzyl-2-dimethylamino-1-( Phenylphenyl)-butanone-1 (Irgacure 369, Ciba Company) and 50ml of dry treated ethyl acetate, heated to the substrate dissolved, slowly added dropwise with dry redistilled neutral dimethyl sulfate 6.51g (0.05mol, pH=6), reflux and stir for 6 hours. After the reaction, the solvent was removed by a rotary evaporator to obtain a viscous crude product N-(2-(N', N'-dimethylamino)-2-benzyl-n-butyryl)phenyl-N-methyl Morpholine sulfate monomethyl ester quaternary ammonium salt, the product is soluble in water. The reaction equation is shown in formula (3).
[0032]
[0033] Formula (3)
Embodiment 2
[0035] Anion exchange: Add 2.58g of the synthesized quaternary ammonium salt intermediate and 50ml of deionized water in a single-necked flask equipped with a stirrer and a constant pressure dropping funnel, and add dropwise 3.8% tetraphenyl at a concentration of 3.8% under stirring Sodium borate aqueous solution 60ml, about 0.007mol sodium tetraphenylborate in total. A white precipitate gradually formed during the dropwise addition. After the dropwise addition, 50ml of deionized water was added, stirred thoroughly, filtered with suction, and rinsed with a small amount of mixed solvent of acetone and water (V / V 1:1). Dry to obtain a light yellow powder, which is determined to be N-(2-(N',N'-dimethylamino)-2-benzyl-n-butyryl)phenyl-N-methylmorpholine tetraphenylboron Quaternary ammonium salt, easily soluble in acetone, acetonitrile, insoluble in water, ethanol and ether. The melting point is 245-146°C, and the melting point of the substrate Irgacure 369 is 117-119°C. The reac...
Embodiment 3
[0039] Add 0.04mol 2-benzyl-2-dimethylamino-1-(morpholinephenyl)-butanone to a three-necked flask equipped with a magnetic stirrer, reflux condenser (with drying tube), thermometer and dropping funnel -1 (Irgacure 369, Ciba Company) and 50ml of dry treated ethyl acetate, heated to the dissolution of the substrate, and slowly added dropwise with dry redistilled neutral diethyl sulfate 7.7g (0.05mol, pH=6 ), reflux stirred and reacted for 6 hours. After the reaction, the solvent was removed by a rotary evaporator to obtain a viscous crude product N-(2-(N', N'-dimethylamino)-2-benzyl-n-butyryl)phenyl-N-ethyl Morpholine sulfate monoethyl ester quaternary ammonium salt, the product is soluble in water. The reaction equation is shown in formula (5).
[0040]
[0041] Formula (5)
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com