Protection and enhancement of erythropoietin-responsive cells, tissues and organs
An erythropoietin, cell technology, applied in the directions of erythropoietin, cytokines/lymphokines/interferons, extracellular fluid diseases, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
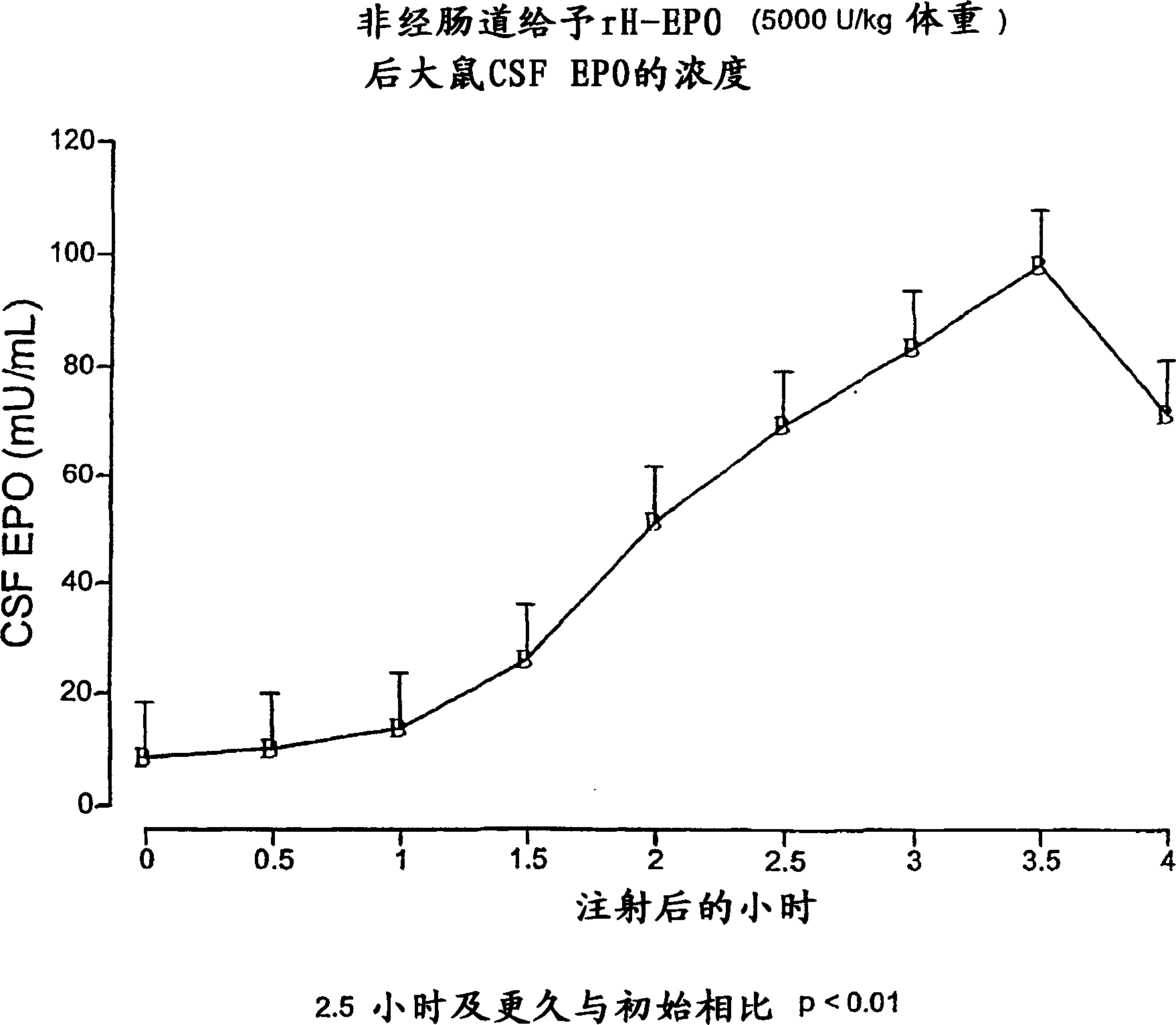
[0175] Erythropoietin crosses the tight blood-cerebrospinal fluid barrier
[0176] Mature male Sprague-Dawley rats were anesthetized and administered recombinant human erythropoietin by intraperitoneal injection. Cerebrospinal fluid (CSF) was sampled every 30 minutes from the cisterna magna until 4 hours, and erythropoietin concentrations were determined using a sensitive specific enzyme-linked immunoassay. like figure 1As shown, the initial erythropoietin concentration in CSF was 8 mU / ml. After a delay of several hours, the measured erythropoietin concentration in CSF started to increase and was significantly different from the initial concentration at a level of p<0.01 at 2.5 hours and later. Peak concentrations of about 100 mU / ml are within the range (0.1-100 mU / ml) known to exert protective effects in in vitro assays. The time to peak occurred at about 3.5 hours, which was significantly delayed from the time to peak serum concentrations (less than 1 hour). The results ...
Embodiment 2
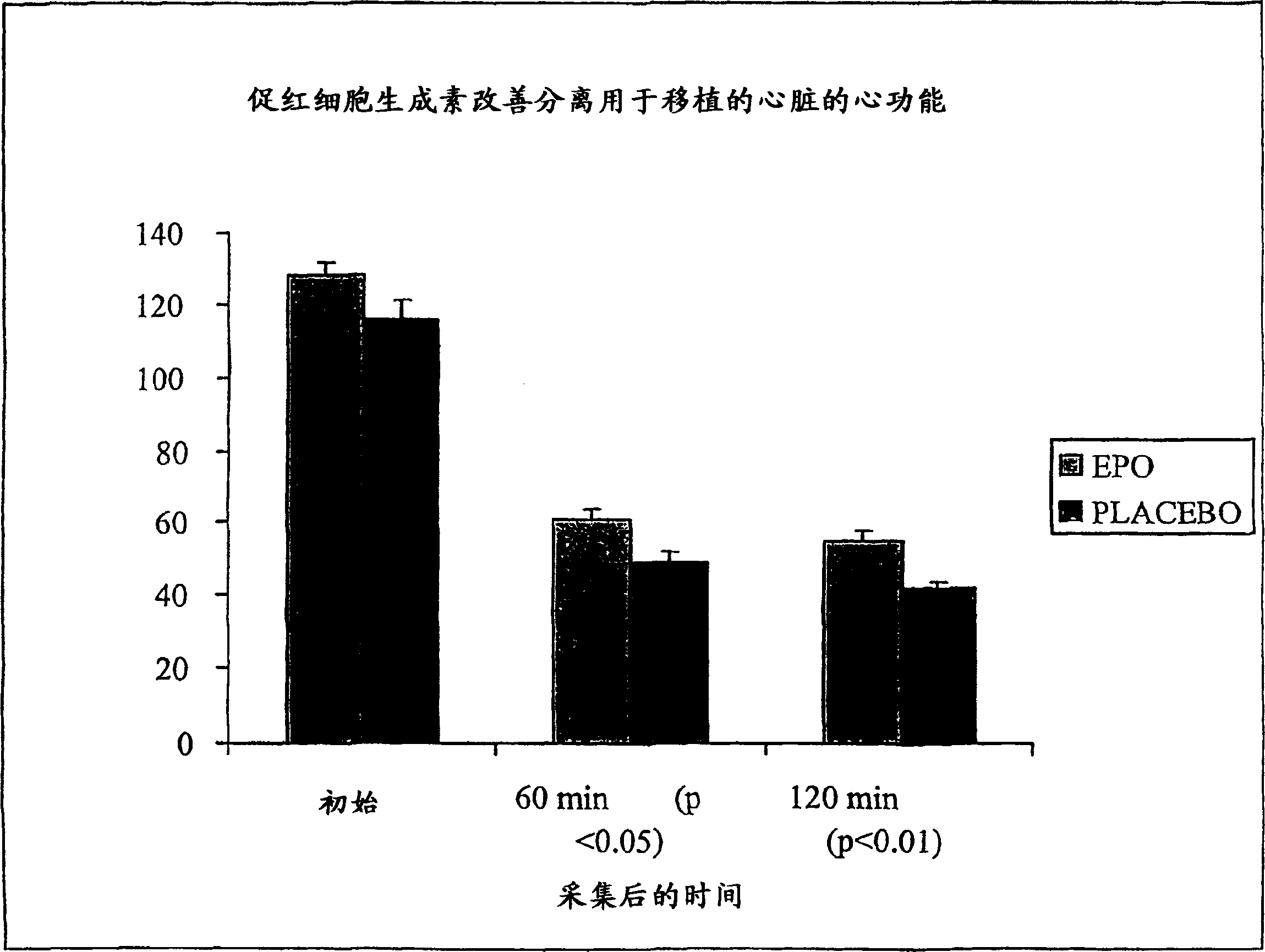
[0178] Maintenance of function in hearts prepared for transplantation
[0179] Wistar male rats weighing 300 to 330 g were given erythropoietin (5000 U / kg body weight) or vehicle 24 h prior to heart removal for ex vivo studies, then followed by Delcayre et al., 1992, Amer. J. Physiol .263: The protocol for H1537-45 was performed. Animals were sacrificed under pentobarbital anesthesia (0.3 mL) and heparinized (0.2 mL) by intravenous injection. The heart was allowed to initially equilibrate for 15 minutes. The left ventricular balloon was then inflated to a volume with an end-diastolic pressure of 8 mmHg. Left ventricular pressure-volume curves can be constructed by inflating the balloon volume in 0.02 ml aliquots. Zero volume is defined as the point at which the left ventricular end-diastolic pressure is zero. When the pressure-volume curve was complete, the left ventricular balloon was deflated to bring the end-diastolic pressure back to 8 mmHg and this control period was ...
Embodiment 3
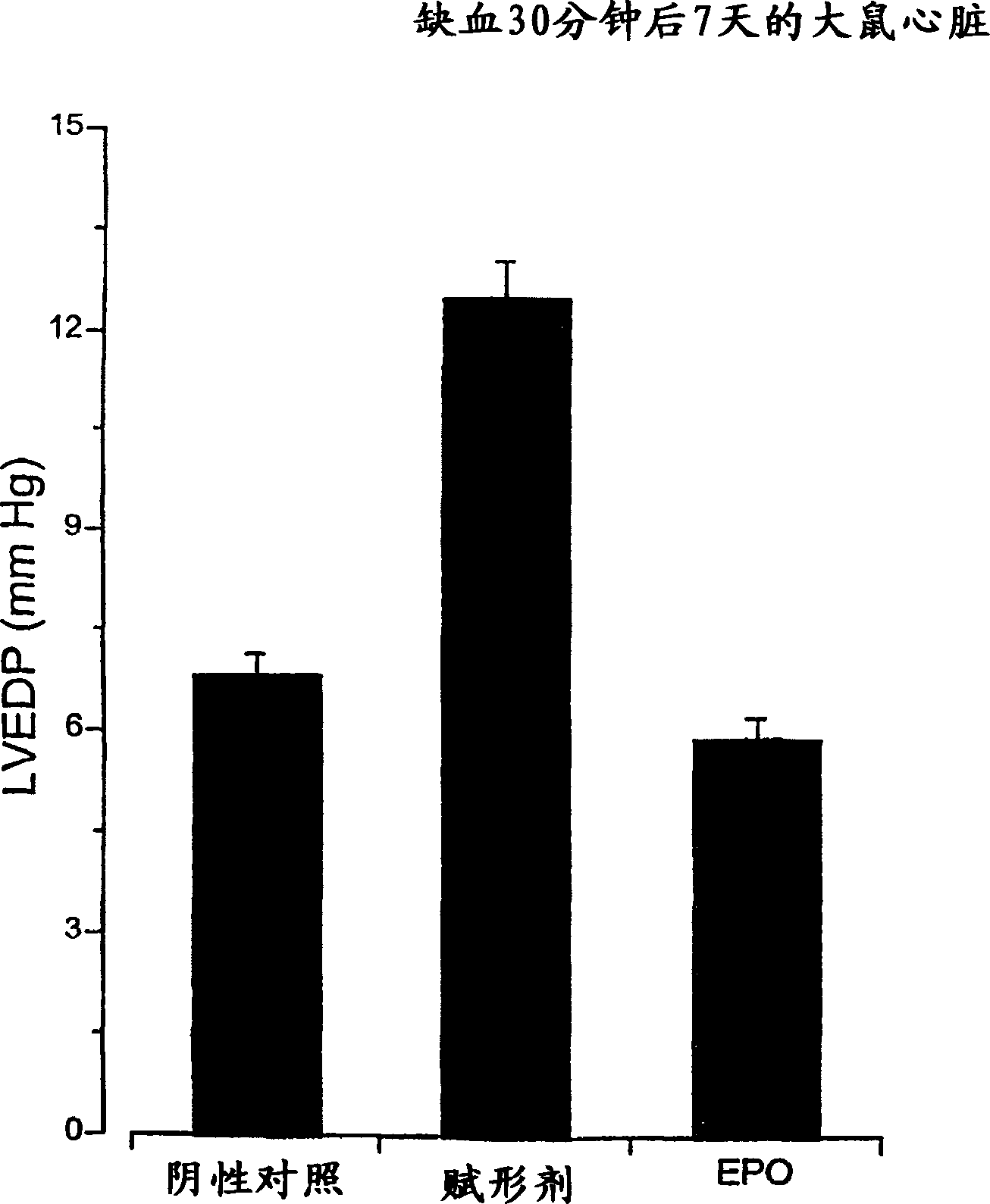
[0182] Erythropoietin protects myocardium from ischemic injury
[0183] Mature male rats administered recombinant human erythropoietin (5000 U / kg body weight) 24 hours earlier were anesthetized and coronary occlusions were prepared. At the beginning of the experiment, erythropoietin was supplemented once, and the left main coronary artery was occluded for 30 minutes and then released. The same dose of erythropoietin was administered daily for one week after treatment. The animals' heart function was then studied. like image 3 As shown, animals that received sham injections (saline) exhibited a large increase in left-sided end-diastolic pressure, showing an enlarged, stiff heart secondary to myocardial infarction. In marked contrast to sham-treated controls, animals receiving erythropoietin did not suffer a decline in cardiac function (significance level of difference at p<0.01).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap