Treatment of ADHD
An indole, acetyl-based technology used in the treatment of ADHD to address irritability, spasticity and depressive symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
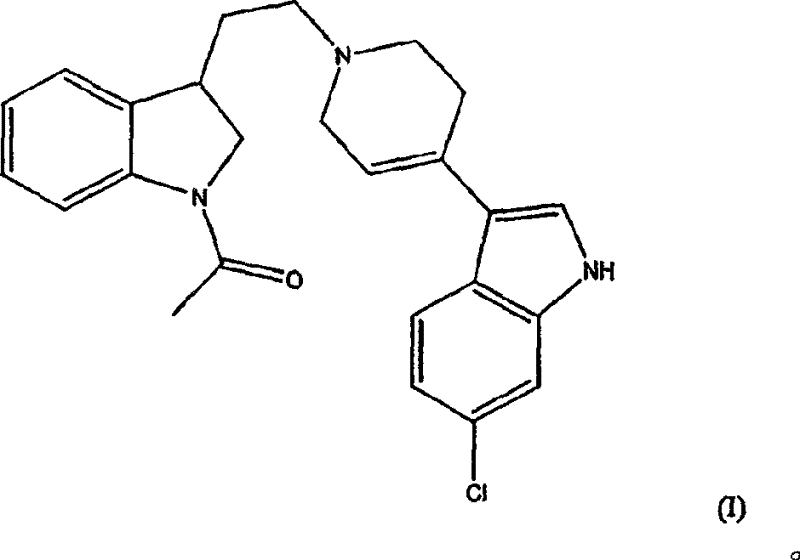
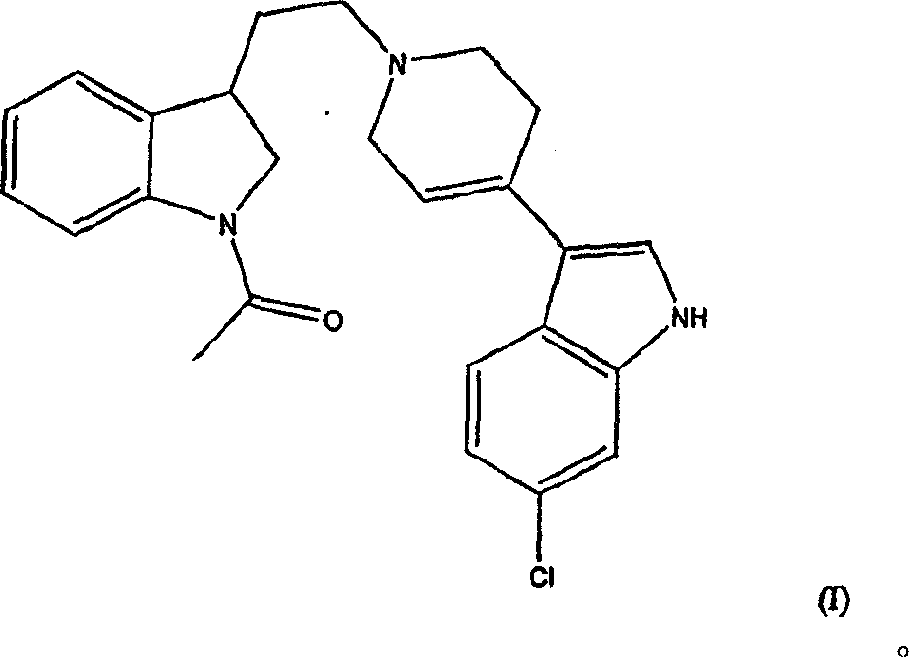
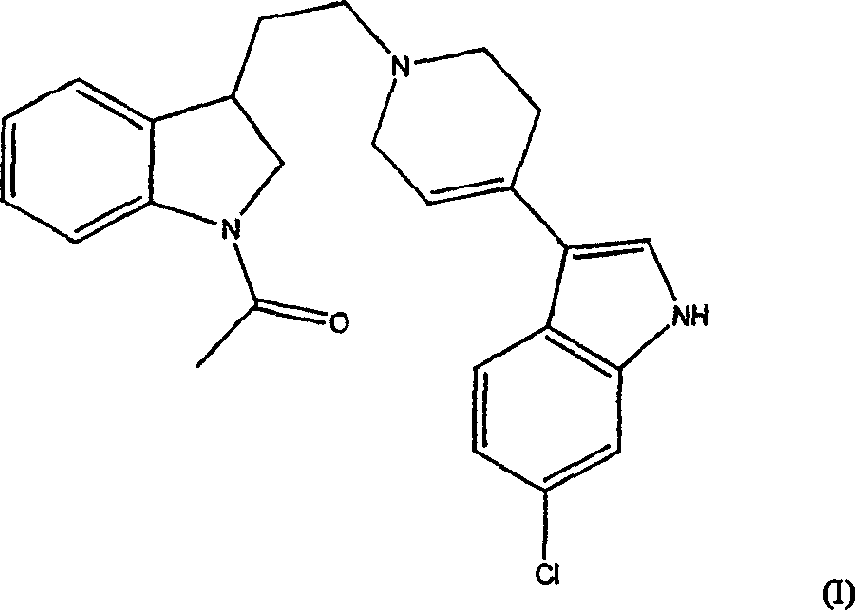
[0014] Compound 3-[1-[2-(1-acetyl-2,3-dihydro-1H-indol-3-yl)ethyl]-1,2,3,6-tetrahydropyridin-4-yl ]-6-Chloro-1H-indole and its enantiomers were first disclosed in WO98 / 28293. The application also contains evidence that the compound is a potent dopamine D 4 Ligand data.
[0015] The present invention includes the use of the racemate and each enantiomer of the compound of formula (I) for the treatment of attention deficit hyperactivity disorder.
[0016] Compounds of formula (I) and their enantiomers and pharmaceutically acceptable salts can be prepared as described in WO98 / 28293, see in particular Examples 20 and 34.
[0017] The compound --(S)-(+)-3-[1-[2-(1-acetyl-2,3-dihydro-1H-indol-3-yl)B base]-1,2,3,6-tetrahydropyridin-4-yl]-6-chloro-1H-indole was tested in which said DAT-KO mice are a kind of Animal models of individual-like behavior and responses to psychostimulants (Gainetdinov et al., Science 1999, 283, 397-401). The compound was found to be effective in this mod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com