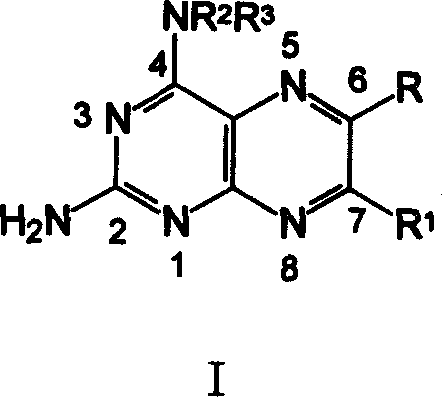
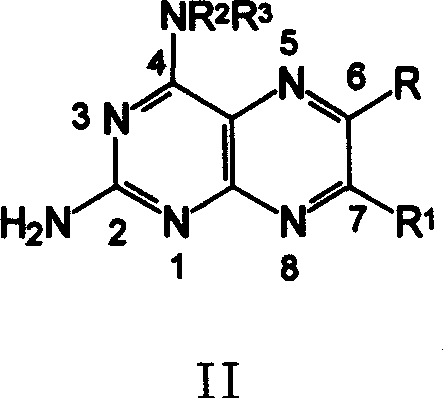
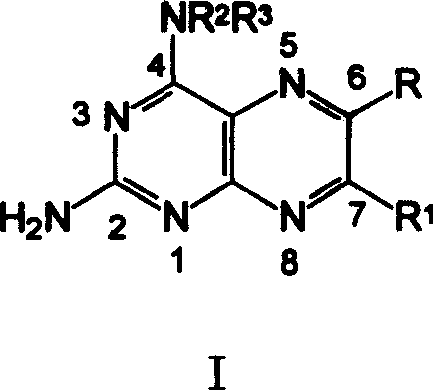
Pteridine derivatives with nitric oxide synthase inhibitor function
A compound, pteridine technology, applied in the field of pteridine derivatives, can solve the problem of lack of NOS isomerase inhibitory selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0088] 1. Compound preparation
[0089] 2-amino-4-[N-(ethyl p-aminobenzoate)-yl]-6-phenylpteridine ( 6 )
[0090] 0.68g (1.65mmol) compound A (wherein: NR 2 R 3 = HNC 6 h 4 COOC 2 h 5 ) was added into 15 mL of anhydrous methanol to dissolve it completely, and under the conditions of nitrogen protection, stirring and oil bath reflux, dropwise added a solution composed of benzoxime (C, R”=H) 0.37 g (2.48 mmol) and 6 mL of methanol , reflux reaction for 4 hours, stop heating, and after cooling to room temperature, adjust the pH value to 7-8 with ammonia water, solids precipitated, put it in the refrigerator overnight, and filtered to obtain the crude product, which was recrystallized from a mixture of ether and ethanol to obtain 0.22 g of orange-yellow solid 6 , Yield: 34.6%, m.p.275~277℃. 1 H-NMRδ: 9.32 (s, 1H, 7-H), 9.23 (s, 1H, 4-NH), 8.13-7.97 (m, 6H, Ph-H), 7.55 (m, 3H, Ph-H), 6.06 (bs, 2H, 2-NH 2 ), 4.39 (q, J=6.9Hz, 2H, CH 2 ), 1.41(t, J=6.9Hz, 3H, CH 3 ).ESI-M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com