Use of alkannin in preparing medicine for treating tumor disease
A shikonin and chemotherapeutic drug technology, applied in the direction of antineoplastic drugs, drug delivery, drug combination, etc., can solve the problem of drug efficacy reduction and achieve low toxicity and good clinical tumor chemotherapy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Identification of Drug Resistance Spectrum of Multidrug Resistant Cell K562 / ADR Cell Line
[0030] The multi-drug resistant cell line K562 / ADR was established by Hu Xun et al. in 1995 using the human erythroleukemia cell line K562 induced by the antitumor drug doxorubicin [15] .
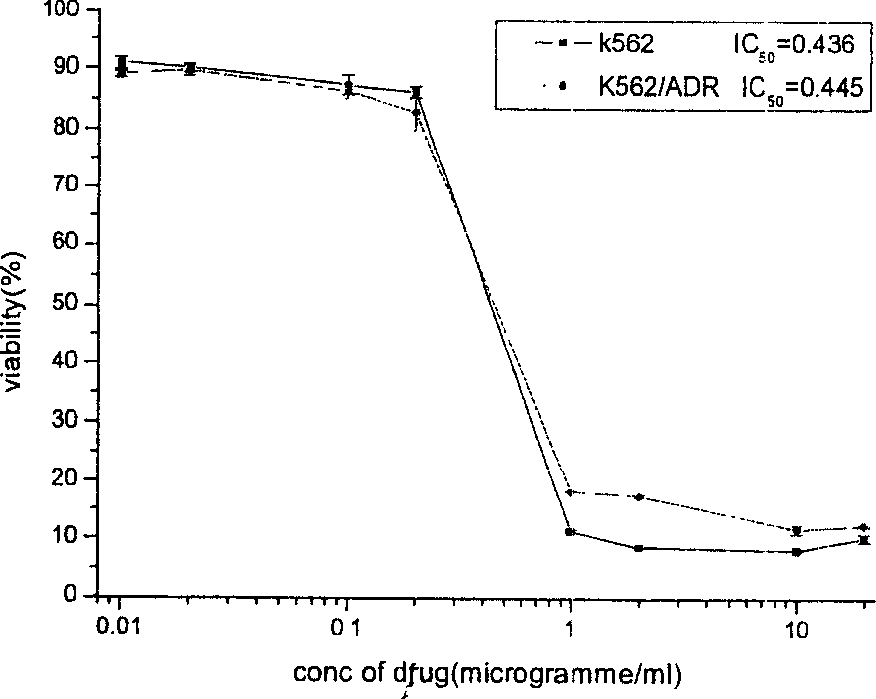
[0031] Determination of resistance of drug-resistant cells to 11 typical antineoplastic drugs by MTT assay
[0032] Take the K562 and K562 / ADR cells in the exponential growth phase with good growth and shape, count them, centrifuge them, and divide them into 8×10 3The concentration of the number of cells / well was inoculated in a 96-well plate, and 100 μl of each well was added with a final concentration of 0.01, 0.02, 0.1, 0.2, 1, 2, 10, and 20 μg / ml of doxorubicin, epirubicin, and daunorubicin, respectively. 11 representative antineoplastic drugs, including vincristine, paclitaxel, methotrexate, fluorouracil, mitomycin C, etoposide, hydroxycamptothecin, homoharringtonine, etc. Th...
Embodiment 2
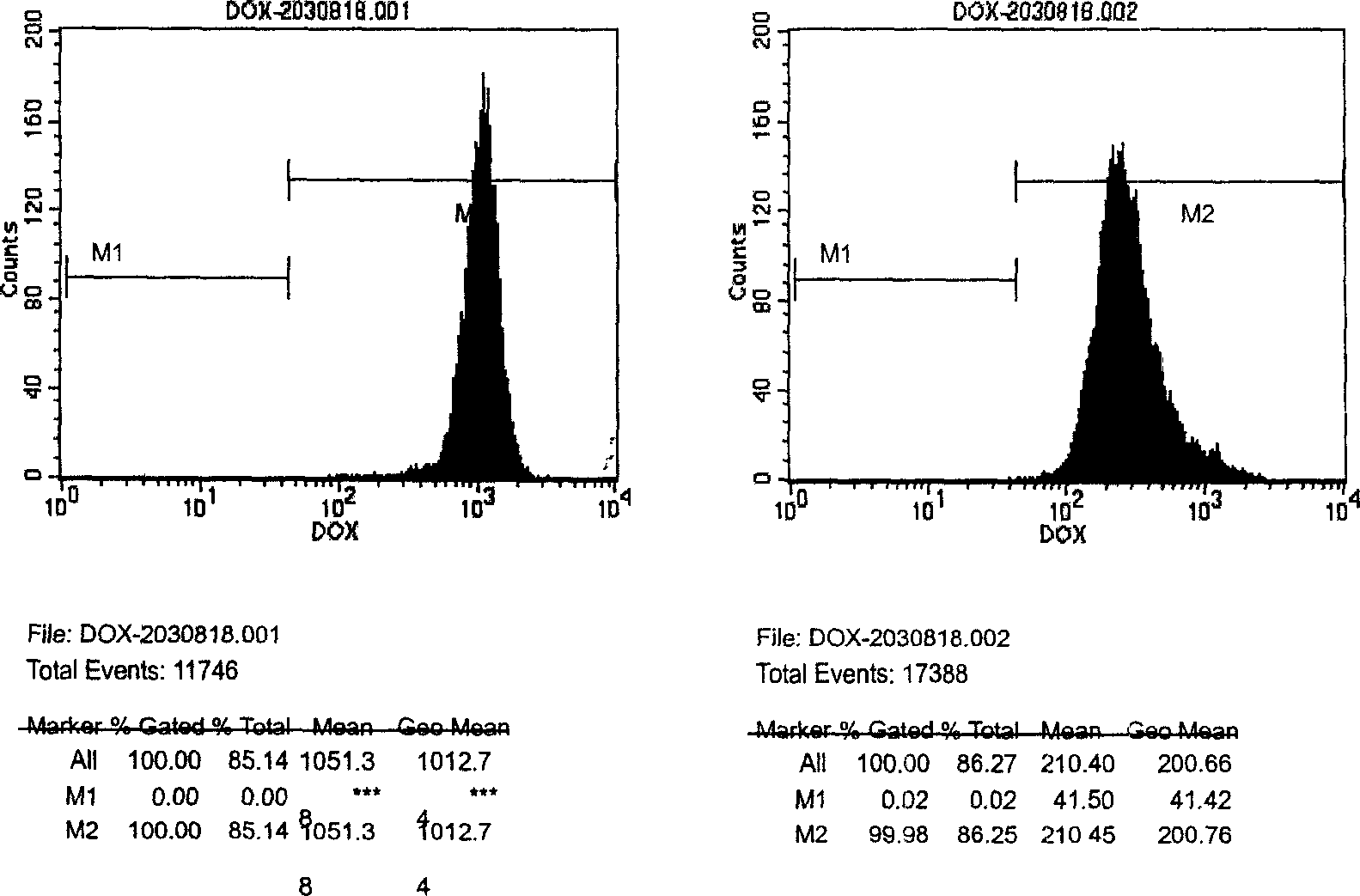
[0054] Example 2 Flow cytometry analysis of the accumulation ability of K562 and K562 / ADR cells to doxorubicin
[0055] Take well-grown K562 and K562 / ADR cells in the exponential growth phase, culture them with doxorubicin at a final concentration of 10 μg / ml in an incubator for 120 min, wash them twice with cold PBS, collect the cells, count them, and use Cold PBS made up 1 x 10 6 Cells / ml cell suspension, stored at 4°C until the sample is loaded, and the fluorescence intensity of doxorubicin in the cell is measured by flow cytometry. The detection excitation wavelength is 488nm, and the acceptance wavelength is 575nm. The arbitrary unit of intensity indicates the relative concentration of doxorubicin in the cell .
[0056] Results: K562 has a strong ability to accumulate doxorubicin. After co-cultivating with 10 μg / ml doxorubicin for 2 hours, the concentration of doxorubicin in K562 cells is about 5 times that of K562 / ADR cells, while K562 / ADR cells are due to Pgp Efflux l...
Embodiment 3
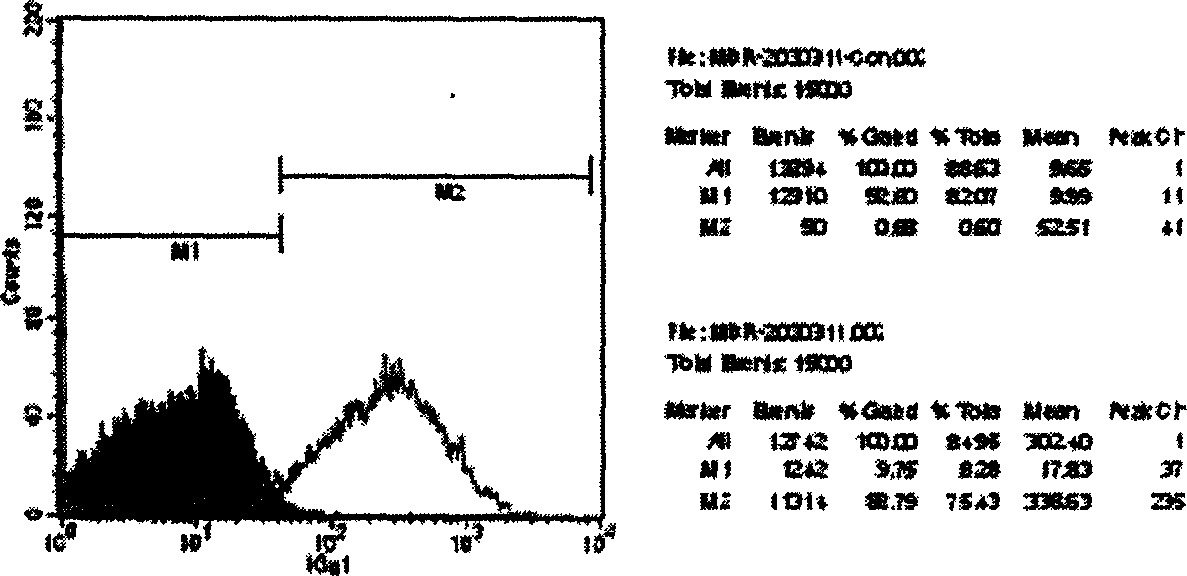
[0057] Example 3 Flow cytometry detection of Pgp expression in K562 / ADR cells
[0058] Take well-grown K562 and K562 / ADR cells in the exponential growth phase, wash them twice with cold PBS, collect the cells, count them, and make 1×10 cells with cold PBS. 6 Add 20 μl of anti-Pgp monoclonal antibody (R-PE-17F9) labeled with PE (Phycoerythrin) to the cell suspension of cells / ml, react at room temperature for 15 minutes in the dark, add PBS and wash twice , load the sample to flow cytometry, and express the expression level of Pgp with the fluorescence intensity.
[0059] Results: In the negative control K562 cells, the proportion of highly expressed Pgp was only 0.68%, while that of drug-resistant K562 / ADR cells was as high as 88.79%. At the same time, the average expression of membrane protein Pgp in drug-resistant cells K562 / ADR was 302.40 / 9.65=31 times that of K562. Therefore, the drug resistance mechanism of K562 / ADR cells is mainly caused by the overexpression of Pgp, se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com