T cell receptor display
A cell receptor, surface display technology, applied in the field of protein-based particles, which can solve problems such as reducing the size of heterologous DNA sequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-A6
[0268] Example 1-A6 Primer Design and Mutagenesis of Tax TCR α and β Chains to Introduce Cysteine Residues Required for New Interchain Disulfide Bond Formation
[0269] To mutate A6 Tax threonine 48 of exon 1 in TRAC*01 to cysteine, the following primers were designed (mutations are indicated by lowercase letters):
[0270] 5'-C ACA GAC AAA tgT GTG CTA GAC AT (SEQ ID 35)
[0271] 5'-AT GTC TAG CAC Aca TTT GTC TGT G (SEQ ID 36)
[0272] To mutate A6 Tax serine 57 of exon 1 in TRBC1*01 and TRBC2*01 to cysteine, the following primers were designed (mutations are indicated by lowercase letters):
[0273] 5'-C AGT GGG GTC tGC ACA GAC CC (SEQ ID 37)
[0274] 5'-GG GTC TGT Gca GAC CCC ACT G (SEQ ID 38)
[0275] PCR mutagenesis
[0276] Expression plasmids containing A6 Tax TCR α or β chain genes were mutated as described below using α chain primers or β chain primers, respectively.
[0277] Mix 100 ng of plasmid with 5 µl of 10 mM dNTPs, 25 µl of 10xPfu buffer (Stratagene), ...
Embodiment 2
[0278] Example 2 - Construction of phage display vector and cloning of A6 TCRα and β chains into the phagemid vector
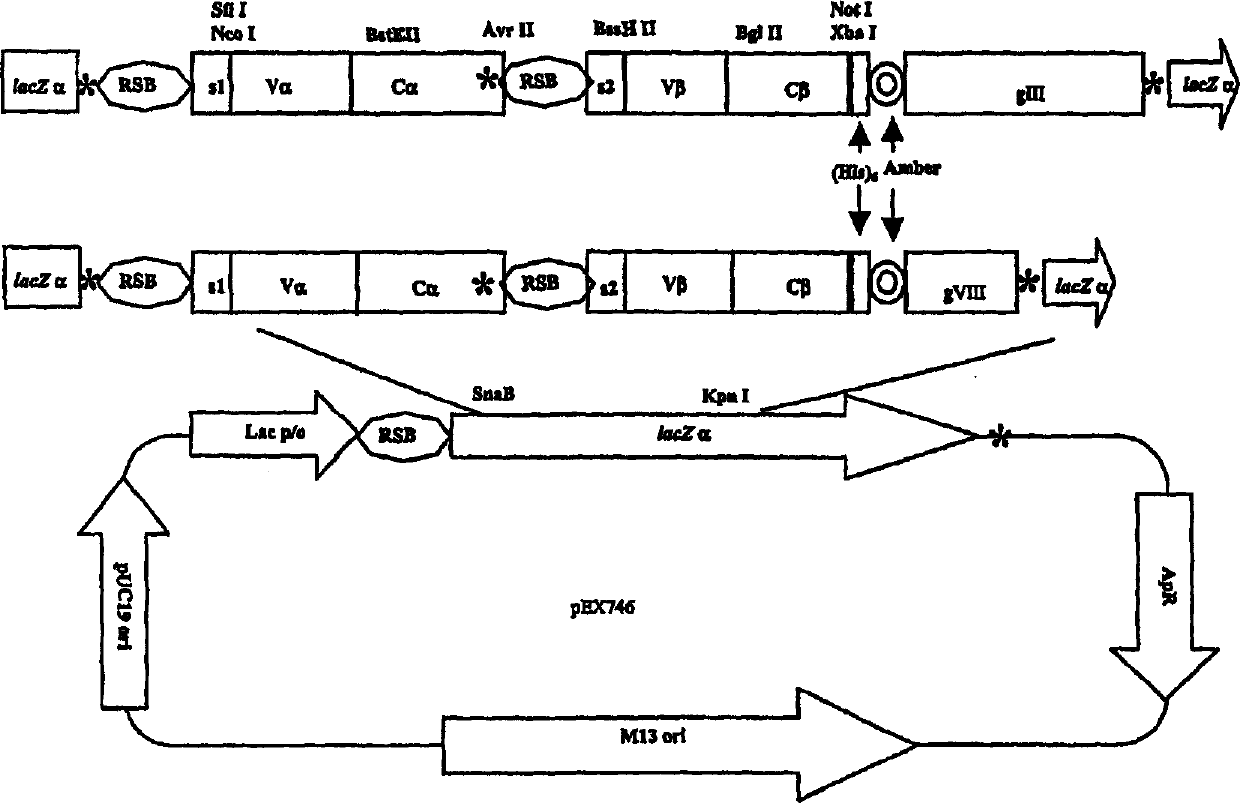
[0279] To display the heterodimeric A6 TCR containing non-native interchain disulfide bonds on filamentous phage particles, a phagemid vector was constructed to express the heterodimeric A6 TCR containing non-native interchain disulfide bonds and phage Fusion protein composed of coat protein. These vectors contain a pUC19 origin, an M13 origin, a bla (ampicillin resistance) gene, Lac promoter / operator and a CAP binding site. The design of these vectors is outlined in Figure 3, which depicts vectors encoding A6 TCR beta chain-g3 or A6 TCR beta chain-g8 fusion proteins in addition to soluble A6 TCR alpha chain. A6 TCR α and β chains prepared in Example 1 and shown in Figures 1a and 1b containing mutations incorporating additional cysteine residues required for formation of new interchain disulfide bonds were used to generate Source of A6 TCR α and β chains f...
Embodiment 3
[0284] Example 3-Expression of fusion protein of bacterial coat protein and heterologous dimer A6 TCR in Escherichia coli
[0285] To validate the constructs generated in Example 2, phages displaying non-native interchain disulfides were prepared using the phage previously described for generating scFv displaying antibodies (Li et al., 2000, Journal of Immunological Methods 236:133-146) and subsequent modifications to produce scFv containing non-native interchain disulfides. Keyed A6 TCR phage particles. E. coli XL-1-Blue cells containing the pEX746:A6 phagemid (i.e., the phagemid encoding the soluble A6 TCR alpha chain and the A6 TCR beta chain fused to the phage gIII protein produced as described in Example 2) It was used to inoculate 5 ml of Lbatg (Lennox L medium containing 100 μg / ml ampicillin, 12.5 μg / ml tetracycline and 2% glucose), and then the culture was cultured overnight (16 hours) at 37° C. with shaking. 50 µl of overnight culture was used to inoculate 5 ml of TY...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap