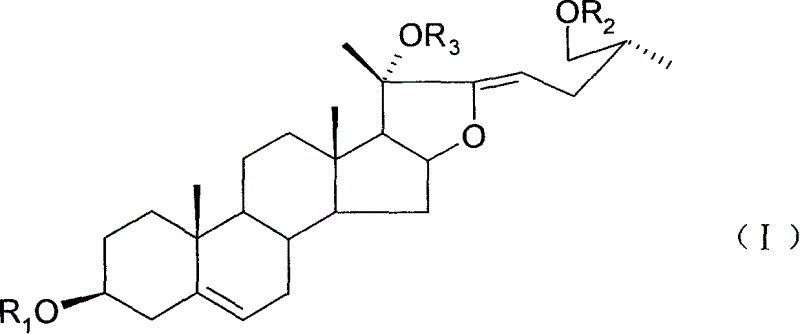
Steroid saponin of alpha, beta double bond structure outside furan nucleus, preparation method and application
A steroidal saponin and furan ring technology, applied in the field of medicinal chemistry, can solve the problems of poor solubility and low bioavailability, and achieve the effects of reducing weight, reducing infarct size, and inhibiting platelet aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
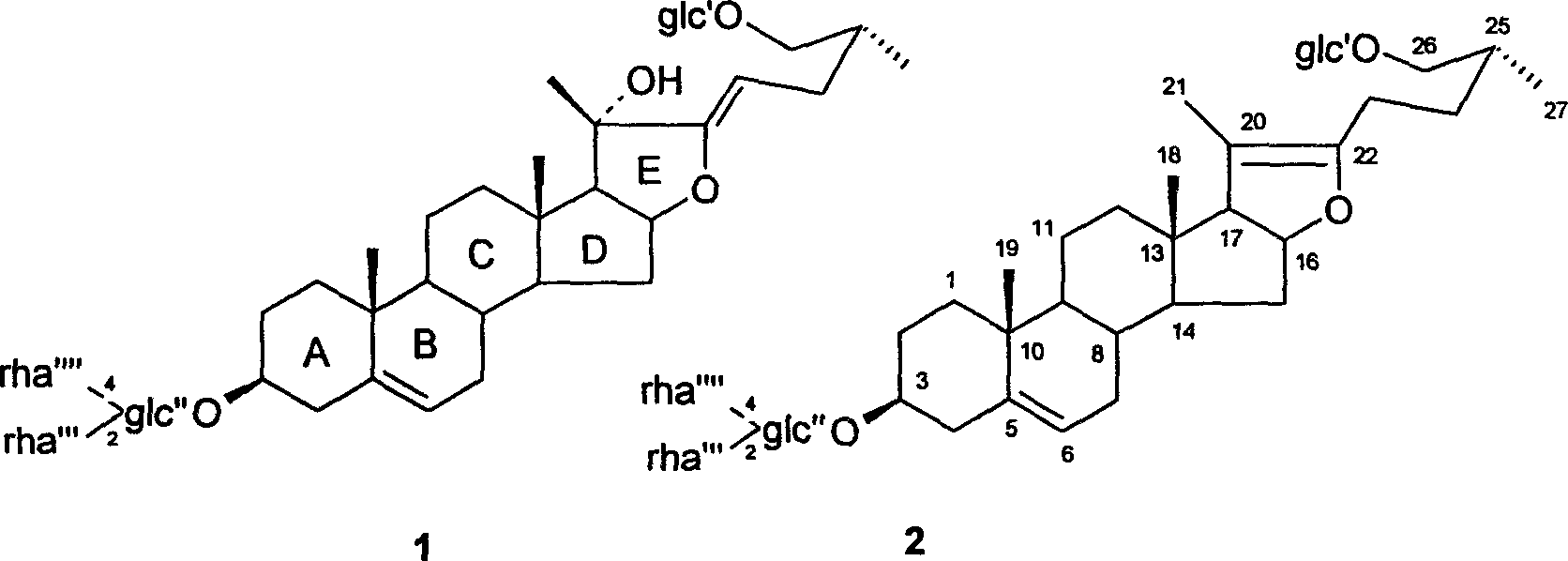
[0018] Example 1 26-O-β-D-glucopyranosyl-3β, 20α, 26-triol-25(R)-Δ 5,22 -Diene-furostan-3-O-{[α-L-rhamnopyranosyl(1→2)]-[α-L-rhamnopyranosyl(1→4)]-β- Isolation and structure identification of D-glucopyranoside (Compound 1).
[0019] Take 500g of total dioscin saponins, disperse it in 5000ml of water, and sequentially extract with ethyl acetate (3500ml×4) and n-butanol (3500ml×5) to obtain ethyl acetate extract (45g) and n-butanol extract (230g). The fraction of n-butanol was subjected to silica gel column chromatography with chloroform-methanol-water (9:1:0.1-0:1:0.1) gradient elution, and was divided into 20 fractions. Take the 17th component (40g) and divide it into 5 sections with resin MCI, take the 4th section (2.8g) and separate it with silica gel column chromatography, elute with chloroform-methanol / 4:1, and finally pass through a reversed-phase silica gel column Purification by chromatography afforded compound 1 (15 mg).
[0020] m.p.186-188°C.
[0021] [α] D 22 ...
Embodiment 2
[0041] Example 2 3β, 20α, 26-triol-25(R)-Δ 5,22(23) Preparation of -diene-furostan-26-O-β-D-glucopyranoside
[0042] 26-O-β-D-glucopyranosyl-3β, 20α, 26-triol-25(R)-Δ 5,22 -Diene-furostan-3-O-{[α-L-rhamnopyranosyl(1→2)]-[α-L-rhamnopyranosyl(1→4)]-β- D-Glucopyranoside (compound 1) was mixed with dilute hydrochloric acid, reacted at 60°C for 40 hours, the reaction pH was 6.2, and then extracted with ethyl acetate, evaporated to dryness under reduced pressure to obtain 3β, 20α, 26-tri Alcohol-25(R)-Δ 5,22(23) -diene-furosta-26-O-β-D-glucopyranoside (compound 3).
[0043] ESI-MS:
[0044] 615[M+Na] +
[0045] 591[M-H] -
[0046] 429[M-H-glc] -
[0047] IR(KBr): 3432 (broad, associative multi-OH), 2926 (CH 2 ), 1634 (CH 3 ), 1450, 1386, 1040-1055, 913, 889. 1 H NMR (400MHz, C 5 D. 5 N): δ H 4.81 (1H, d, J=7.9Hz, 1-H-glc'), 3.96 (1H, m, 26-H), 3.64 (1H, m, 26-H), 1.72 (3H, s, 21-CH 3 ), 1.02 (3H, d, J=10.8Hz, 27-CH 3 ), 1.00 (3H, s, 19-CH 3 ), 0.85 (3H, s, 18-CH ...
Embodiment 3
[0049] Example 3 26-O-β-D-glucopyranosyl-3β, 20α, 26-triol-25(R)-Δ 5,22(23) Preparation of -diene-furosta-3-O-α-L-rhamnopyranosyl (1→2)-β-D-glucopyranosyl
[0050] According to the method similar to Example 2, 26-O-β-D-glucopyranosyl-3β, 20α, 26-triol-25(R)-Δ 5,22 -Diene-furostan-3-O-{[α-L-rhamnopyranosyl(1→2)]-[(α-L-rhamnopyranosyl(1→4)]-β -D-Glucopyranoside (compound 1) mixed with dilute hydrochloric acid to prepare 26-O-β-D-glucopyranosyl-3β, 20α, 26-triol-25(R)-Δ 5,22(23) -diene-furosta-3-O-α-L-rhamnopyranosyl(1→2)-β-D-glucopyranosyl (compound 4).
[0051] ESI-MS:
[0052] 901[M+H] +
[0053] 899[M-H] -
[0054] 753 [M-H-rha] -
[0055] IR (KBr): 3436 (broad, associative multi-OH), 2926 (CH 2 ), 1632 (CH 3 ), 1385, 1039-1054, 910, 886. 1 H NMR (400MHz, C 5 D. 5 N): δ H 6.40 (1H, br s, l-H-rha), 5.28 (1H, br s, 6-H), 5.05 (1H, d, J=6.8Hz, 1-H-glc″), 4.83 (1H, d, J = 7.7Hz, l-H-glc'), 3.93 (1H, m, 26-H), 3.65 (1H, m, 26-H), 1.75 (3H, d, J = 6.4Hz, rha-CH 3...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap