Finely prepared controlled release preparation for coronary disease and its preparation process
A technology for controlled release preparation and coronary heart, which is applied in the field of refined coronary controlled release preparation and its preparation technology, can solve the problems of large dosage of refined coronary heart tablet and backward technological conditions, and achieves improvement of curative effect, reduction of taking times, and carrying handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
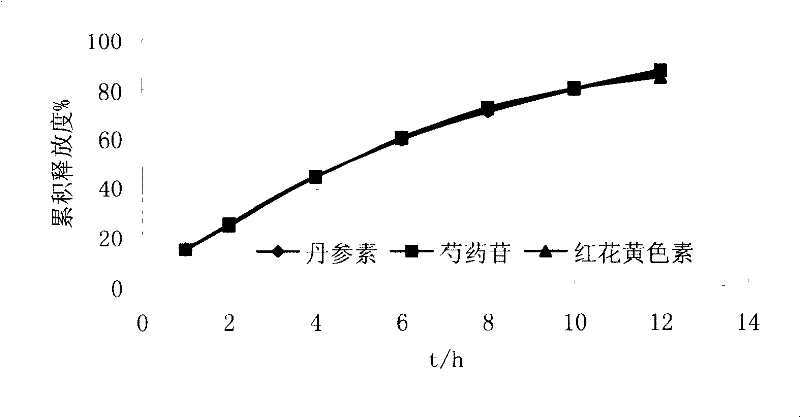
[0033] Extract and separate the various medicinal materials in the refined Guanxin formula, for example: extract the effective fraction group mainly composed of Danshensu by water extraction and alcohol precipitation method and / or other methods; use water extraction and alcohol precipitation method and / or different concentrations of ethanol (including After extracting with water), use macroporous resin separation method and / or other methods to extract the effective fraction group mainly based on paeoniflorin; use water extraction and alcohol precipitation method and / or different concentrations of ethanol (including water) to extract and use macroporous resin separation method and / or other methods to extract the effective part group mainly based on ferulic acid; after extraction with water extraction and alcohol precipitation method and / or different concentrations of ethanol (including water), use macroporous resin separation method and / or other methods to extract safflower Yell...
Embodiment 2
[0052] Each medicinal material in the refined Guanxin prescription extracts and separates with reference to embodiment 1, adopts the tablet (also can be capsule) that the known method of pharmaceutical industry is made to contain following component by weight percentage:
[0053] Tablet Prescription:
[0054] Salvia Extract 23.0%
[0055] Paeoniae Rubra Extract 5.4%
[0056]Chuanxiong Extract 1.2%
[0057] Safflower Extract 11.5%
[0058] Aroma essential oil-β-cyclodextrin inclusion complex 7.9%
[0059] Bergamot Extract 0.7%
[0060] Hypromellose K4M 8.9%
[0061] Sodium chloride 10.0%
[0062] Lactose 10.0
[0063] Polyoxyethylene (PEO) 20.4%
[0064] Magnesium Stearate 1.0%
[0065] Proper amount of ethanol solution
[0066] Coating Solution Prescription:
[0067] Acetate 75%
[0068] PEG-4000 25%
[0069] Using film coating technology, the refined coronary heart is made into a double-layer osmotic pump controlled release tablet, and the drug-containing layer i...
Embodiment 3
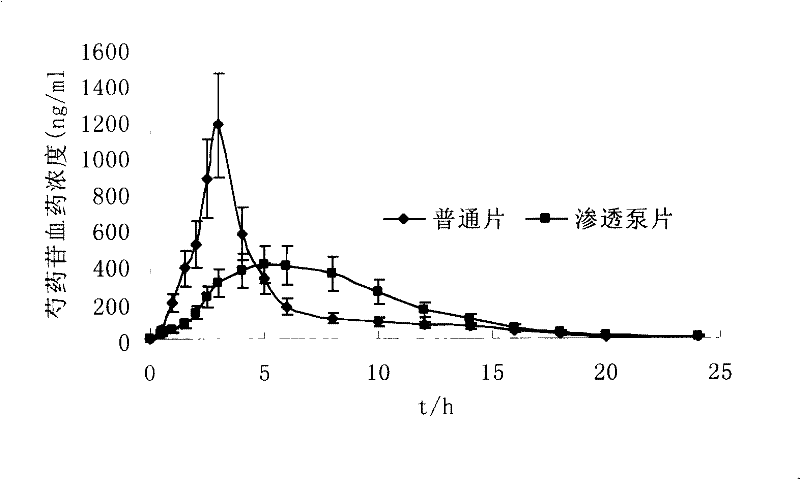
[0071] The refined Guanxin osmotic pump tablet prepared in Example 1 and the refined Guanxin ordinary tablet of the same dose were used to conduct pharmacokinetic studies in vivo with Beagle dogs. The relative bioavailability of paeoniflorin was 98.7%, and the blood drug concentration curve in the body was shown in attached figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com