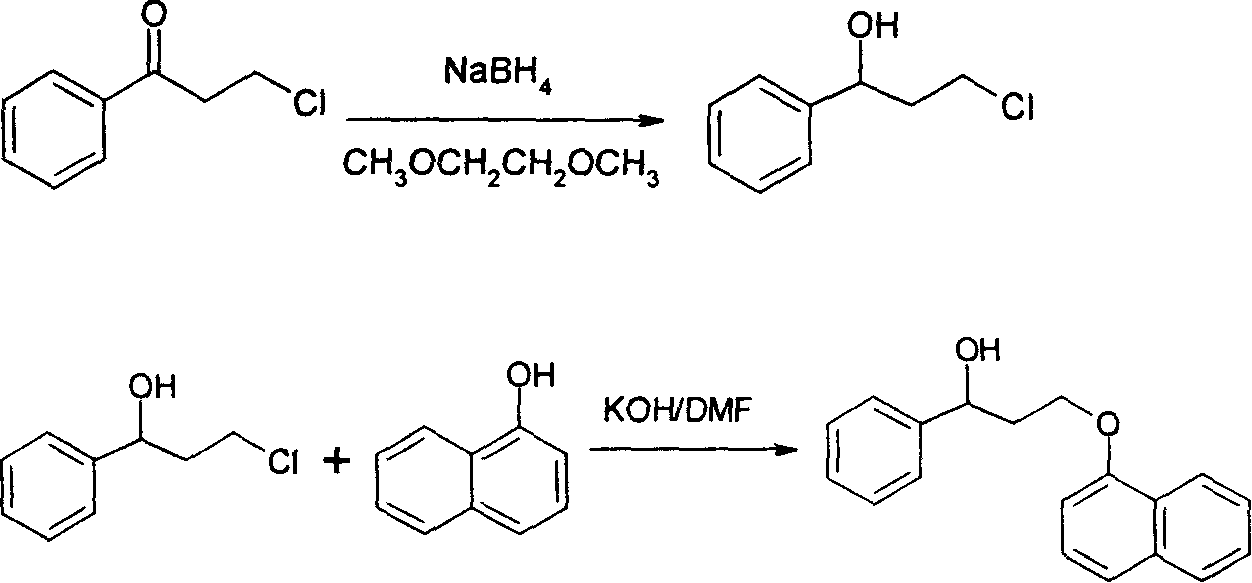
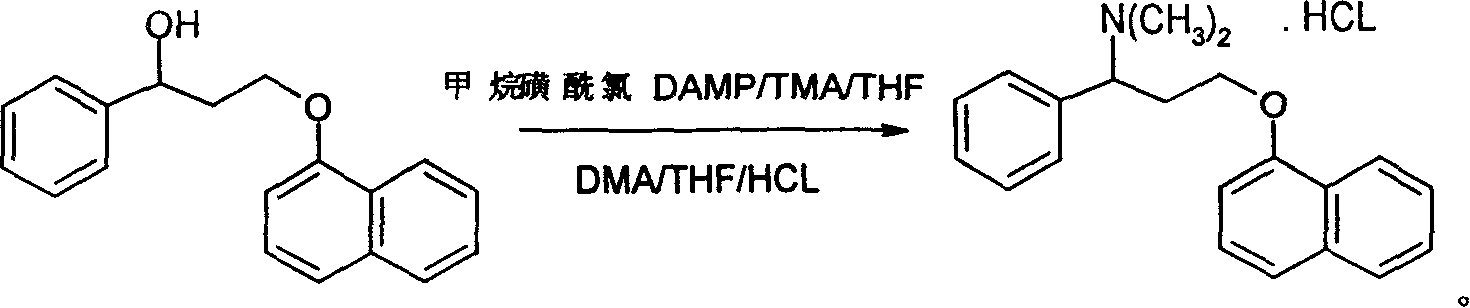
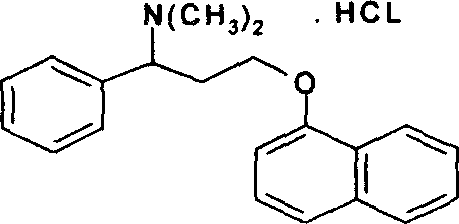
Synthetic method for dapoxetine
A technology for dapoxetine and a synthesis method, which is applied in the field of medicinal chemistry, can solve the problems of harsh reaction conditions, difficult procurement of raw materials, and complicated reaction steps, and achieves the effects of simplifying separation steps, simplifying reaction procedures, and reducing raw material costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 27
[0023] NN-Dimethyl-1-phenyl-3-(1-naphthyloxy)propylamine oxalate, melting point 116-118°C.
[0024] Analyze and press C 23 h 25 NO 5 calculate:
[0025] Theoretical values: C 69.86, H 6.37, N 3.54
[0026] Experimental values: C 70.02, H 6.37, N 3.43
example 28
[0028] NN-Dimethyl-1-phenyl-3-(1-naphthyloxy)propylamine p-benzenesulfonate, melting point 98-110°C, decomposed.
[0029] Analysis and calculation according to C28H31NO4S:
[0030] Theoretical values: C 70.40, H 6.54, N 2.93
[0031] Experimental values: C 70.29, H 6.55, N 3.16
example 36
[0033] (+)NN-Dimethyl-1-phenyl-3-(1-naphthyloxy)propylamine tartrate
[0034] 32.44 g (0.106 mol) of NN-dimethyl-1-phenyl-3-(1-naphthyloxy)propylamine was dissolved in 75 ml of ethanol, and 15.94 g (0.106 mol) of (+)-tartaric acid was dissolved in 650 The solutions in mL of water were combined. The mixture was left at room temperature overnight and the precipitated solid was vacuum filtered. The title compound of this example was finally isolated by multiple recrystallizations, m.p. 94-96°C.
[0035] Analyze and calculate according to C25H29NO7:
[0036] Theoretical values: C 65.92, H 6.42, N 3.08
[0037] Experimental values: C 65.92, H 6.48, N 3.00
[0038] other information:
[0039]
[0040] US Patent: 5,292,962
[0041]
[0042] Although the starting materials produced in the above methods are different, the reaction conditions are harsh, the raw materials are not easy to purchase, and the reaction steps are cumbersome.
Invention content:
[0043] The techn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com