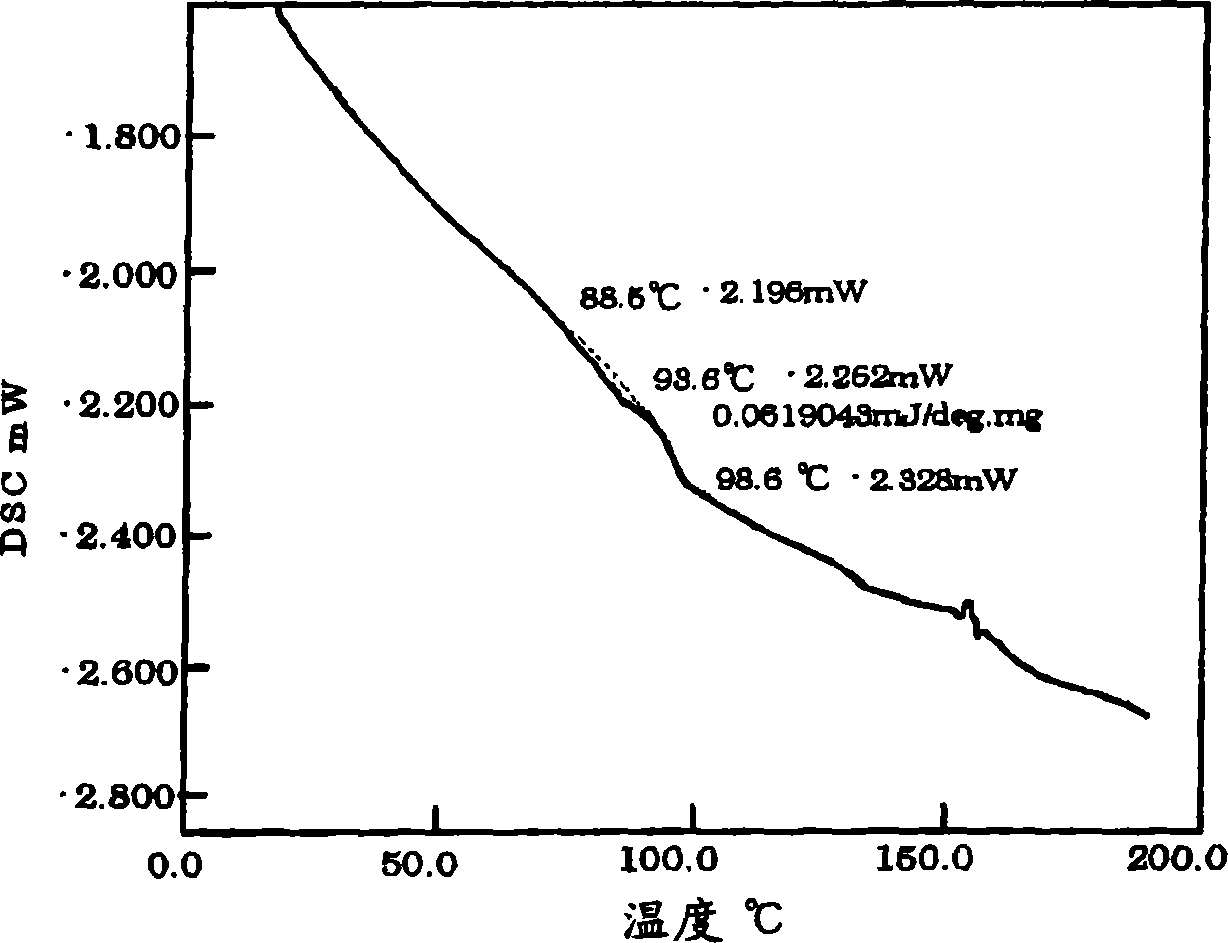
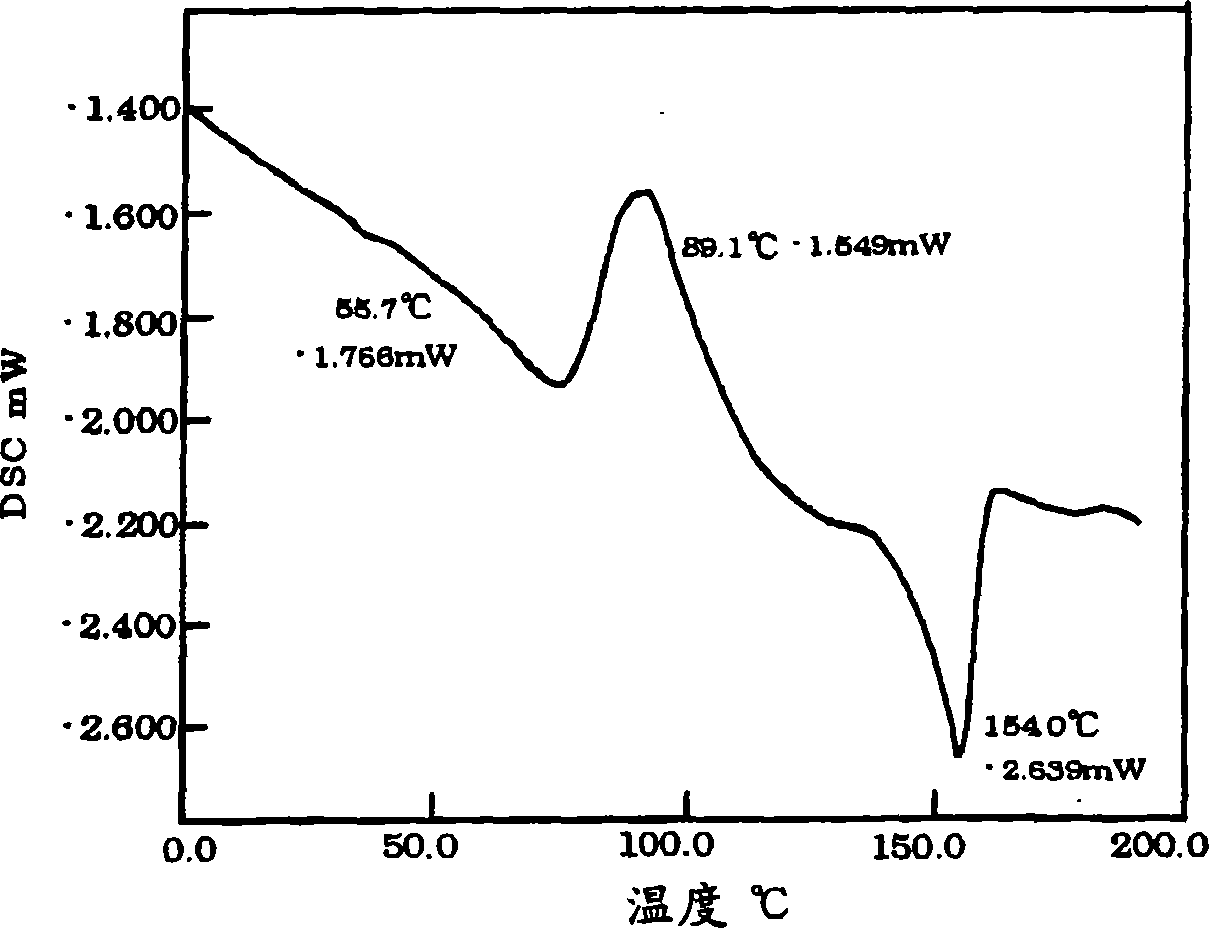
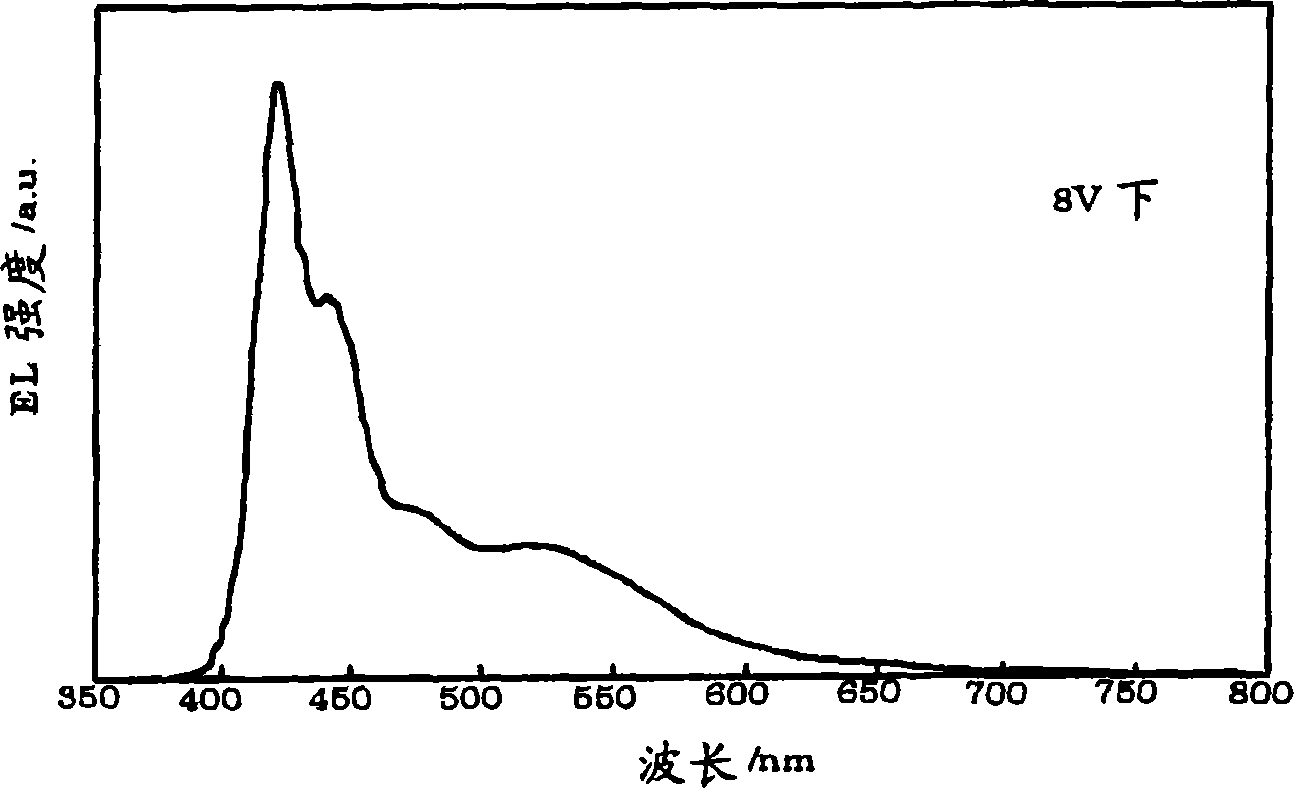
Electroluminescent polymers, organic EL devices and displays
A luminescence and polymer technology, applied in electroluminescent light sources, electrical components, electric light sources, etc., can solve the problems of thermal properties such as glass transition temperature reduction, component life reduction, and changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0056] The present invention will be described more specifically by way of examples below.
reference example 1
[0058] (Synthesis of 2,7-dibromo-9,9-dioctylfluorene)
[0059]
[0060] Add 10.0g (30.9mmol) 2,7-dibromofluorene, 19.7g (102.0mmol) 1-bromooctane, 25ml dimethyl sulfoxide, 24.9g (623mmol ) sodium hydroxide, 50ml of water, heated to 80°C. After confirming the dissolution of 2,7-dibromofluorene, 608 mg (2.66 mmol) of benzyltriethylammonium chloride was added, followed by heating and stirring for 20 hours.
[0061] The obtained reaction solution was extracted with hexane, and the extract was dried, and the hexane was distilled off, and then excess 1-bromooctane was distilled off under reduced pressure under heating. Next, the resulting residue was purified by column chromatography (carrier: silica gel, elution solvent: hexane), and 2,7-dibromo-9,9-dioctylfluorene was isolated as colorless crystals (yield: 14.3 g ( 26.1 mmol), yield 84.5%). The resulting compound was identified by 1 H-NMR, 13 C-NMR performed.
[0062] 1 H-NMR (CDCl 3 , δ): 7.58-7.40 (M, 6H), 1.90 (t, J ...
reference example 2
[0065] (Synthesis of 2,7-dibromo-9,9-bis(2-ethylhexyl)fluorene)
[0066]
[0067] Add 29.3g (90.4mmol) 2,7-dibromofluorene, 75ml dimethyl sulfoxide, 60.0g (311mmol) 1-bromo-2-ethylhexane, 150ml 12.5M sodium hydroxide in a 1000ml eggplant-shaped flask Aqueous solution was stirred, and 1.20 g (5.27 mmol) of benzyltriethylammonium chloride was added thereto. At this point the organic phase was reddish purple. Next, the mixture was stirred at 90°C for two nights, extracted with diethyl ether, and the extract was washed with water and dried.
[0068] Concentrate the dry extract, add 50ml dimethyl sulfoxide, 29.2g (151mmol) 1-bromo-2-ethylhexane, 100ml 12.5M aqueous sodium hydroxide solution to the concentrated solution, stir, add 1.20g (5.27 mmol) benzyltriethylammonium chloride. The mixture was then stirred at 90° C. for 4 days, at which time the organic phase was reddish-purple. Then it was stirred at 90°C for two nights, extracted with diethyl ether, washed and dried.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com