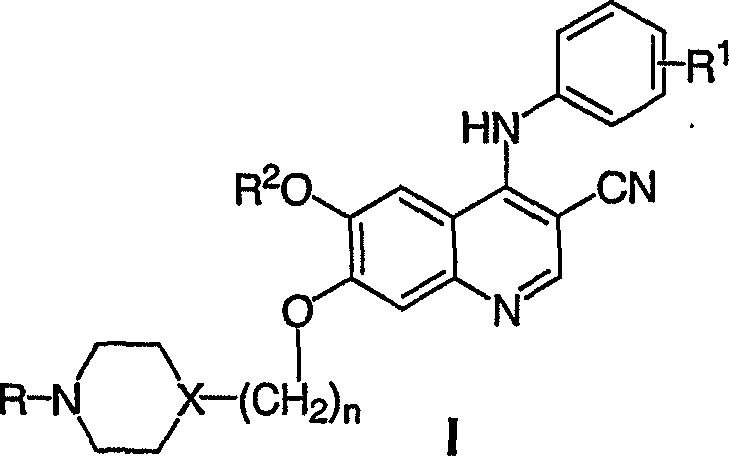
4-anilino-3-quinolinecarbonitriles for the treatment of chronic myelogenous leukemia (CML)
The technology of quinoline nitrile and quinoline is applied in the field of 4-anilino-3-quinoline nitrile for the treatment of chronic myeloid leukemia (CML), and can solve the problems of weak patient response and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The preparation of formula I compound is in literature [Boschelli, D.H., et al., J.Med.Chem., 44,3965 (2001), Boschelli, D.H., et al., J Med.Chem., 44,822 (2001), Boschelli , D.H., et al., Bioorg.Med.Chem.Lett., 13, 3797 (2003), Boschelli, D.H., et al., J.Med.Chem., 47, 1599 (2004) and Ye, F. et al., 221th National Meeting of the American Chemical Society, San Diego, CA (April 2001)].
[0062] The present invention will be more fully described in conjunction with the following specific examples, which should not be construed as limiting the scope of the invention.
[0063] Materials and methods:
[0064] Src kinase assay, homogeneous solution based assay (Lance format)
[0065] Kinase buffer:
[0066] 50mM Hepes pH7.5
[0067] 10mM MgCl 2
[0068] 20 μg / ml BSA
[0069] 0.001% Brij-35
[0070] (For convenience, prepare 2× Kinase Buffer:
[0071] 100mM Hepes, 20mM MgCl 2 , add fresh 40 μg / ml BSA and 0.002% Brij)
[0072] Stop buffer (1:1 continuous addition to ...
example 1
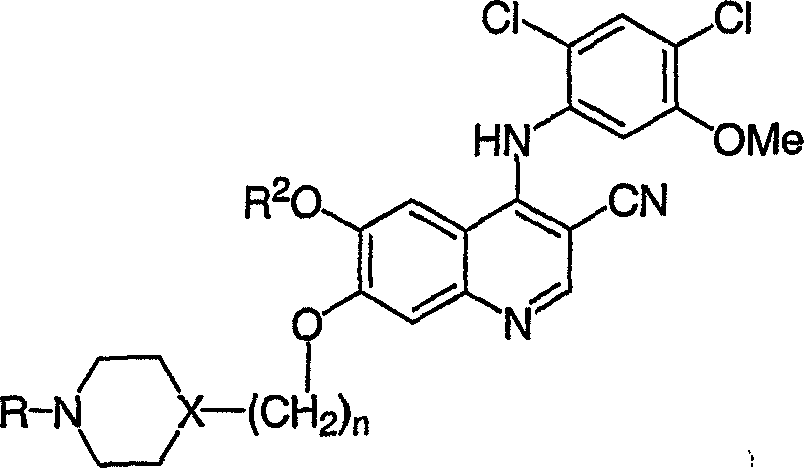
[0120] Example 1 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propoxy ]-3-quinolinenitrile
[0121] mp 116-120°C; MS (ES) m / z 530.2, 532.2 (M+1);
example 2
[0122] Example 2 4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[3-(4-ethyl-1-piperazinyl)propoxy]-6-methoxy yl-3-quinolinecarbonitrile
[0123] mp 102-104°C; MS (ES) m / z 544.3, 546.4 (M+1);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com