Reagent kit for detecting hepatitis B virus e antigen and use
A hepatitis B virus and kit technology, applied in the field of virus immunology detection, can solve the problems of low sensitivity, high false positive rate, limited clinical application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 The establishment of anti-HBeAg hybridoma cell line and the preparation of its monoclonal antibody
[0055] (1) Immunization of animals
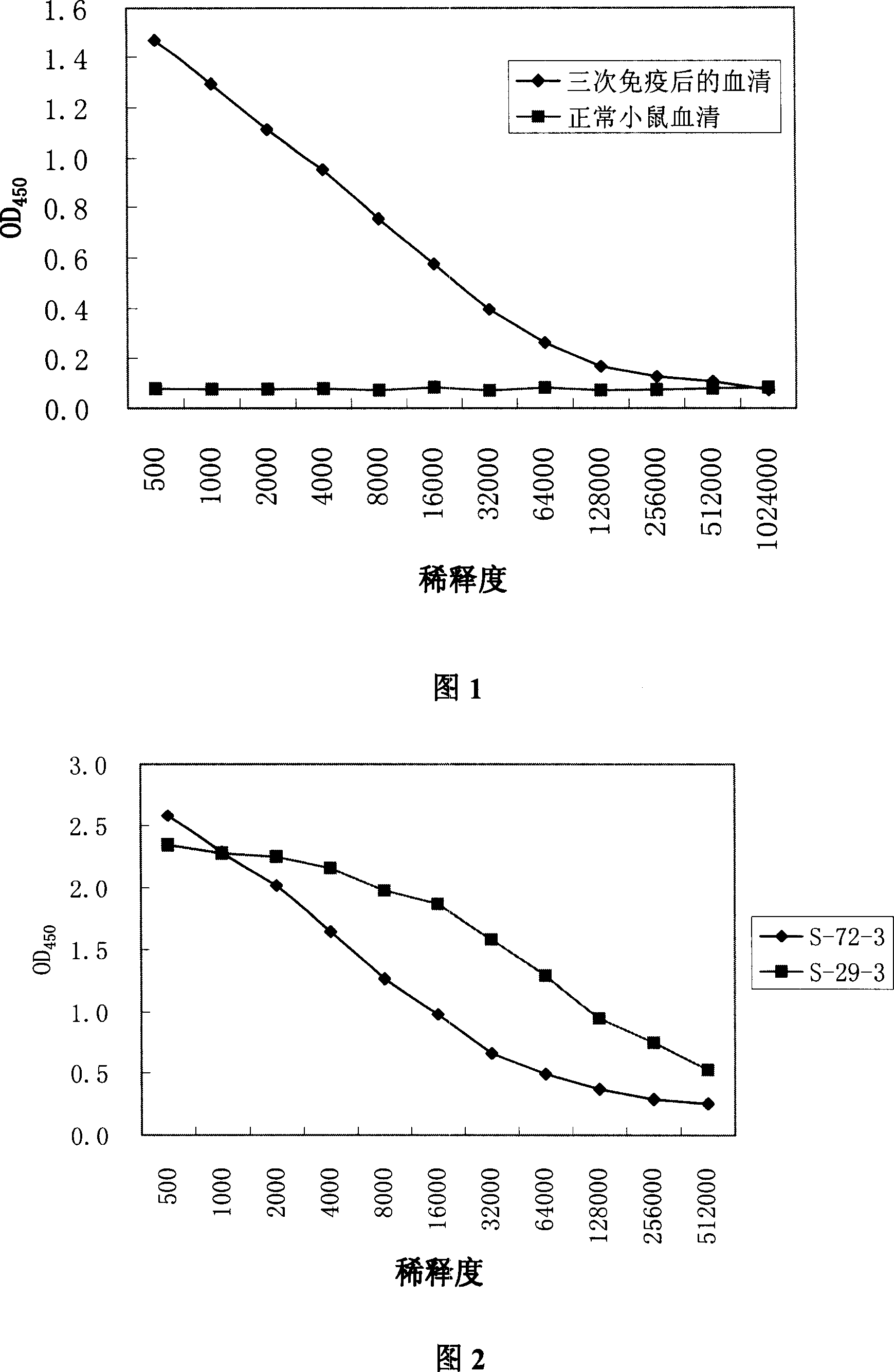
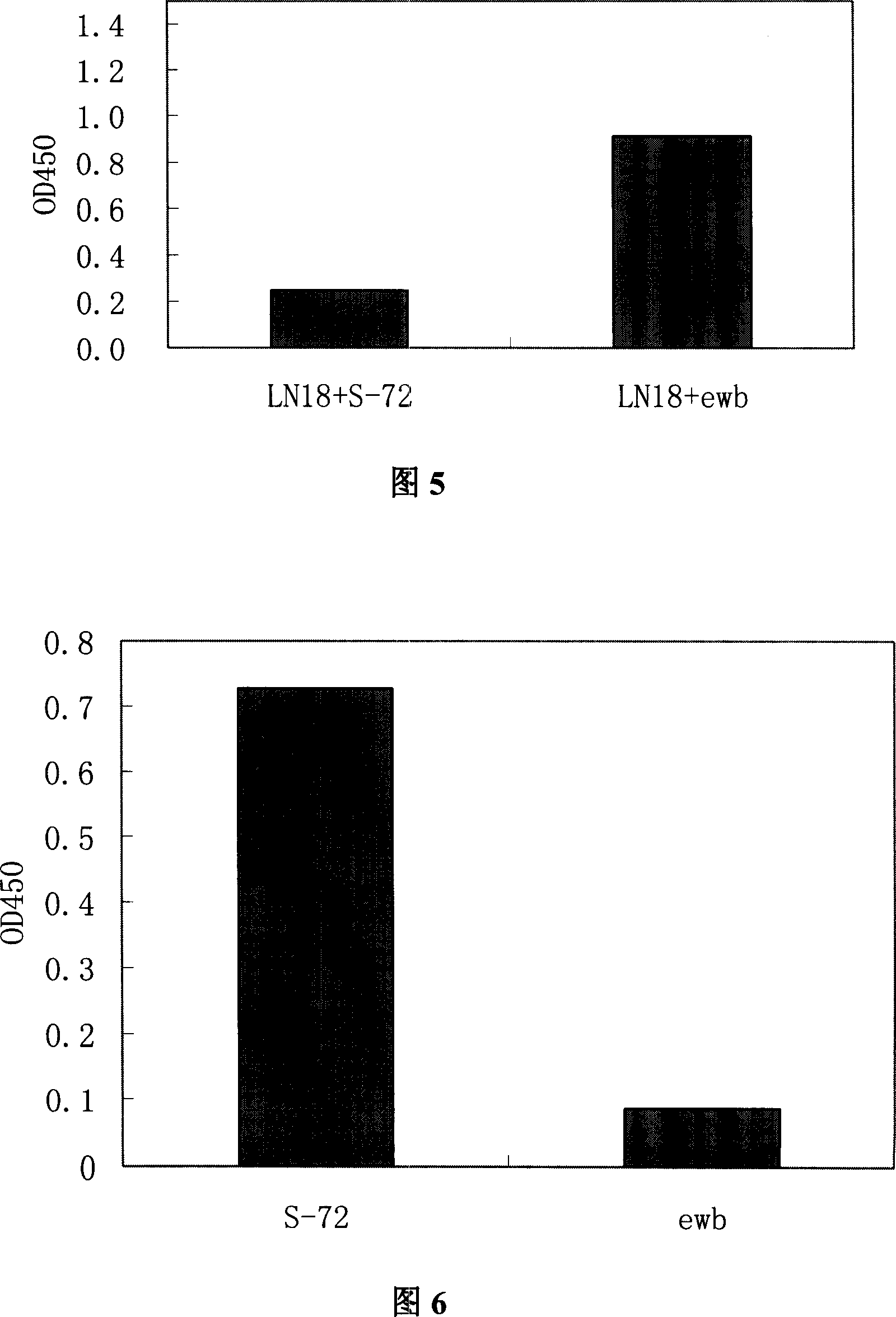
[0056]BALB / C mice were immunized three times with recombinant HBeAg protein. One day before the first immunization, 0.5 mg of antibody ewb (product of Shanghai Kehua Co., Ltd.) recognizing the b epitope of HBeAg was intravenously injected. For the first immunization, Freund's complete adjuvant was mixed with equal amount of HBeAg protein, then emulsified, and injected intravenously, 0.1 mg per mouse, with an injection volume of 0.2-0.3 mL. For the second and third immunizations, use Freund's incomplete adjuvant and equal amounts of HBeAg protein to mix and then emulsify. The immunization method and dosage are the same as the first immunization. Before each immunization of mice, 0.1 mg of HBeAg and 0.5 mg of antibody ewb were mixed and emulsified with an equal amount of adjuvant. Each immunization interval is one month....
Embodiment 2
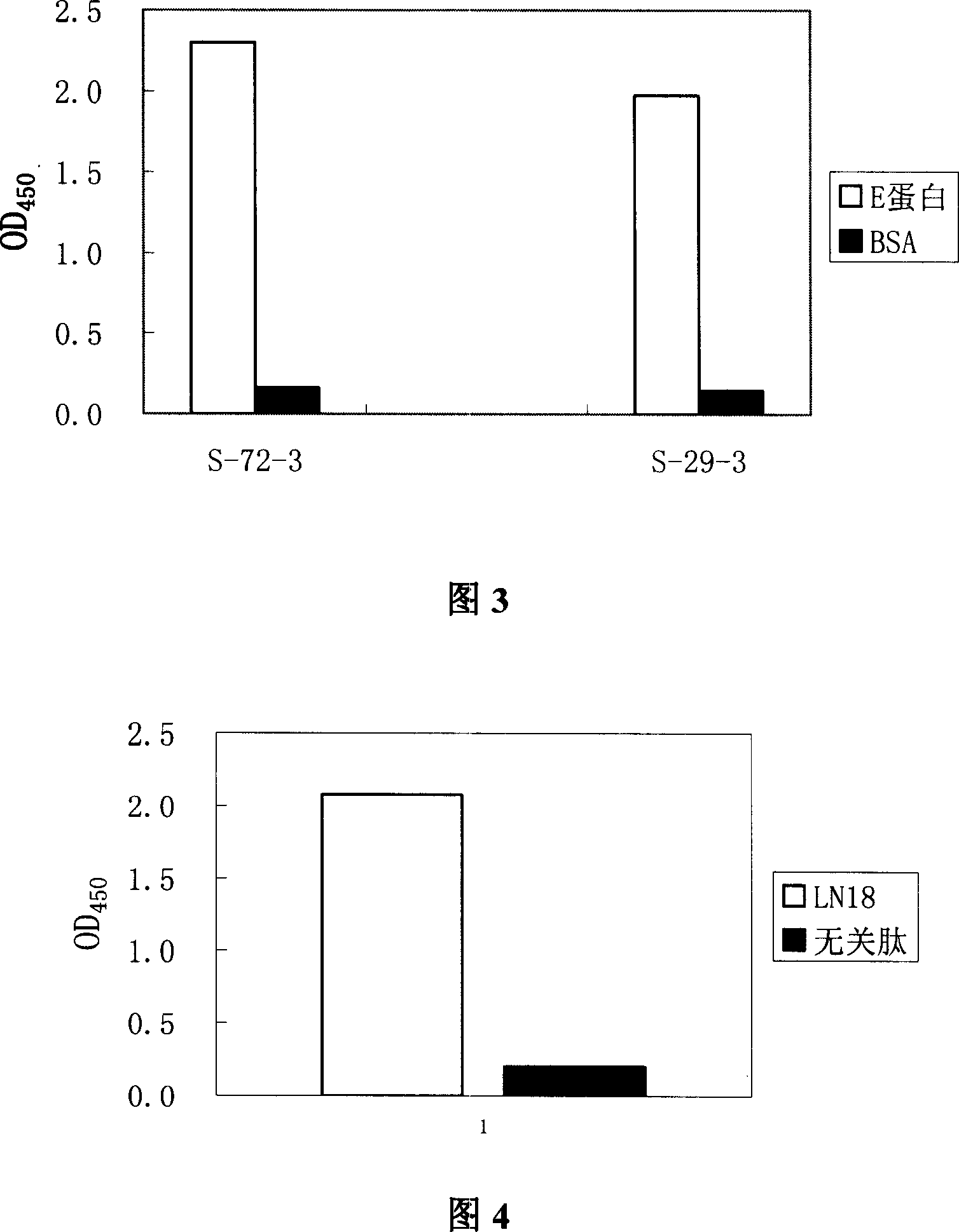
[0063] Embodiment 2 Preparation of anti-HBeAg monoclonal antibody S-29-3
[0064] The positive hybridoma cell line S-29-3 was mixed with 1×10 6 BABL / C female mice sensitized at the age of 8-10 weeks were intraperitoneally injected per mouse. After 7-10 days, the ascites of the mice was collected, and the titer of S-29-3 monoclonal antibody was detected by indirect ELISA method. The ascites was centrifuged at 4°C and 12000rpm for 15 minutes, and 1 part (volume) of ascites was mixed with 2 parts (volume) of 0.06 M pH4.8 acetate buffer, and n-octanoic acid 33 μL / mL was added dropwise with stirring at room temperature, and mixed for 30 minutes at room temperature. Stand at 4°C for more than 2 hours to fully precipitate, then centrifuge at 4°C and 12,000 rpm for 30 minutes, discard the precipitate, filter the supernatant through a sand core funnel, add 1 / 10 volume of 0.1M PBS with pH 7.4, and adjust the pH with 2M NaOH to 7.4. Add 0.277g / mL ammonium sulfate within 30min under ice...
Embodiment 3
[0065] Embodiment 3 Preparation of anti-HBeAg monoclonal antibody S-72-3
[0066] The positive hybridoma cell line S-72-3 was mixed with 1×10 6 BABL / C female mice sensitized in advance at the age of 8-10 weeks were intraperitoneally injected per mouse. After 7-10 days, the ascites of the mice was collected, and the remaining steps were the same as in Example 2 to prepare antibody S-72-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com