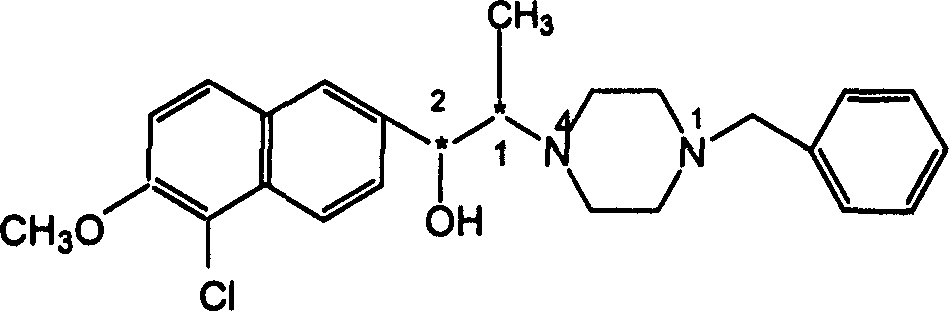
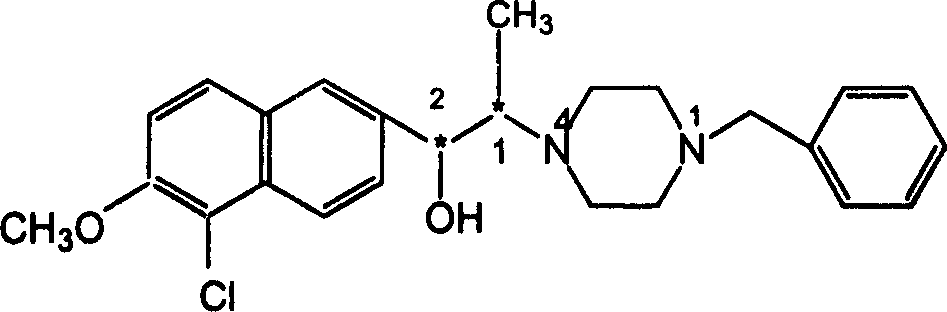
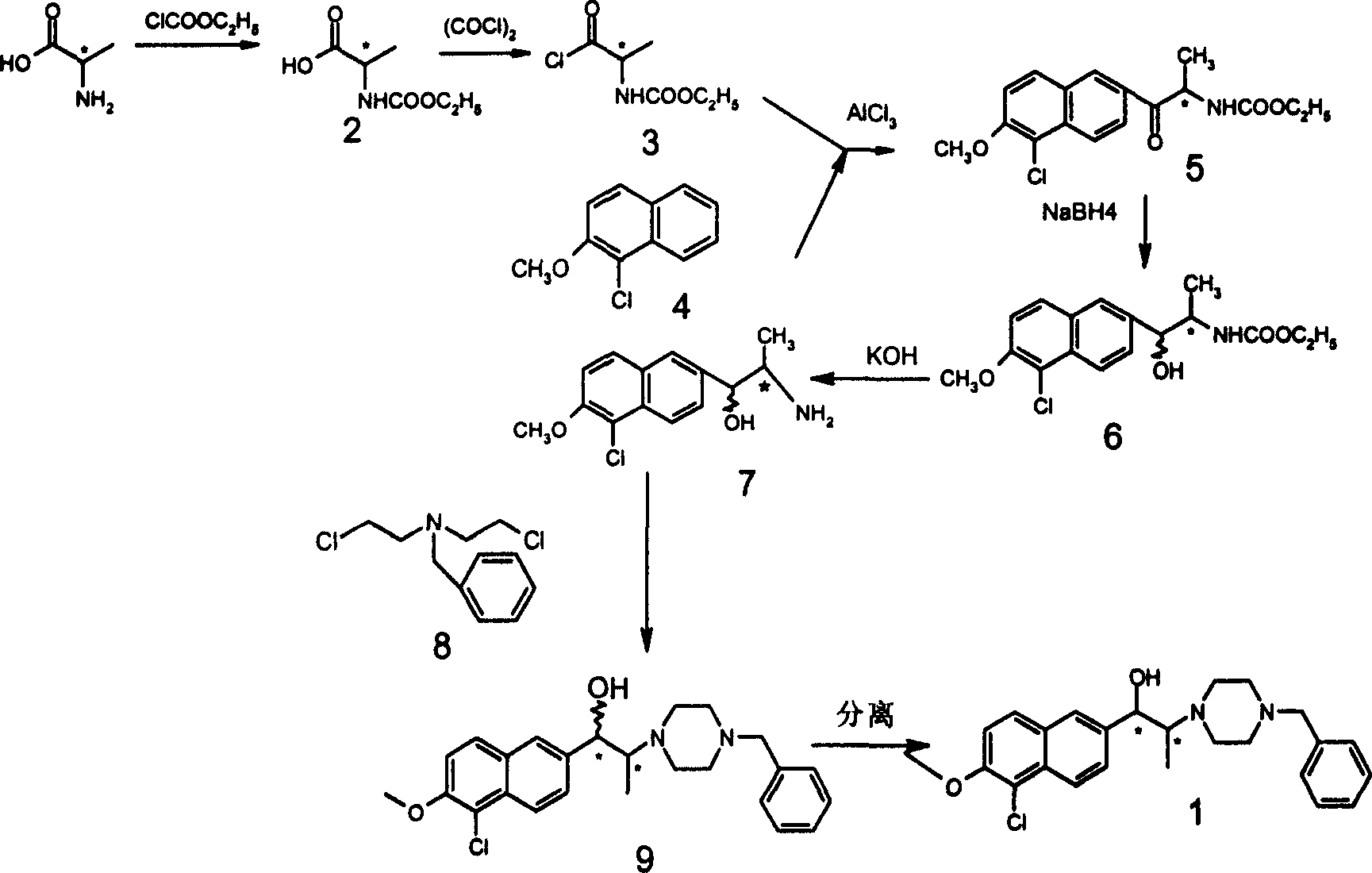
Alkylol piperazine derivative optical isomer or its salt and its application
A technology of optical isomers and derivatives is applied in the application field of preparing antidepressant drugs, and can solve problems such as large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Synthesize (1S, 2R)-SIPIyy24 optical isomers by referring to general methods 1-5
[0060] [α] D 25 =-8.05° (C=1, CH 3 OH); mp: 242-243°C (dec); MS: m / z 425 (M+);
[0061] 1 HNMR (DMSO-d6): δ1.05 (d, 3H, NCHCH 3 ), 3.47-4.28 (m, 9H, NCHCH 3 , piperazine-H), 3.99 (s, 3H, OCH 3 ), 5.59 (d, 1H, PhCHOH, J=3.2), 7.45-8.10 (m, 10H, ArH).
[0062] Elemental Analysis: C 25 h 29 ClN 2 o 2 2HCl 2H 2 Theoretical value of O: C: 56.24%, H: 6.51%, N: 5.25%; measured value: C: 56.04%, H: 6.36%, N: 5.30%.
Embodiment 2
[0064] Synthesize (1R, 2S)-SIPIyy24 optical isomers by referring to general methods 1-5
[0065] [α] D 25 =8.54° (C=1, CH 3 OH); mp: 242-243°C (dec); MS: m / z 425 (M+);
[0066] 1 HNMR (DMSO-d6): δ1.05 (d, 3H, NCHCH 3 ), 3.33-4.33 (m, 9H, NCHCH 3 , piperazine-H), 3.99(s, 3H, OCH 3), 5.64 (d, 1H, PhCHOH, J=3.2), 7.45-8.10 (m, 10H, ArH).
[0067] Elemental Analysis: C 25 h 29 ClN 2 o 2 2HCl 2.5H 2 O Theoretical value: C: 55.31%, H: 6.68%, N: 5.16% Measured value: C: 55.20%, H: 6.77%, N: 5.26%
Embodiment 3
[0069] Synthesize (1S, 2S)-SIPIyy24 optical isomers by referring to general methods 1-5
[0070] [α] D 25 =50.60° (C=1, CH 3 OH); mp: 242-243°C (dec); MS: m / z 425 (M+);
[0071] 1 HNMR (DMSO-d6): δ1.07 (d, 3H, NCHCH 3 ), 3.42-3.81 (m, 9H, NCHCH 3 , piperazine-H), 4.41(s, 3H, OCH 3 ), 4.93 (d, 1H, PhCHOH, J=9.6), 7.54-8.21 (m, 10H, ArH).
[0072] Elemental Analysis: C 25 h 29 ClN 2 o 2 2HCl 2H 2 O Theoretical value: C: 56.24%, H: 6.51%, N: 5.25% Measured value: C: 56.44%, H: 6.21%, N: 5.30%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com