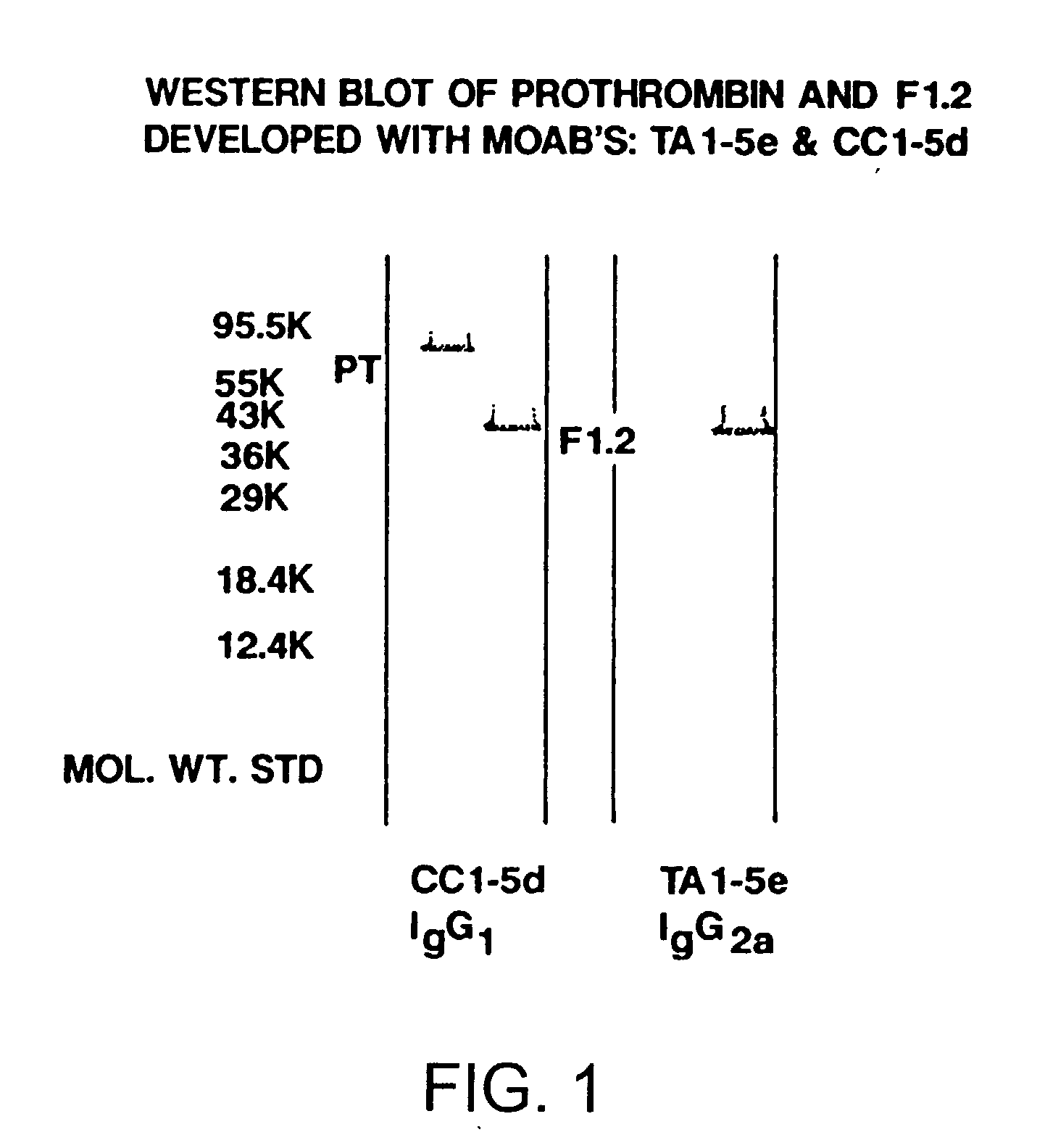
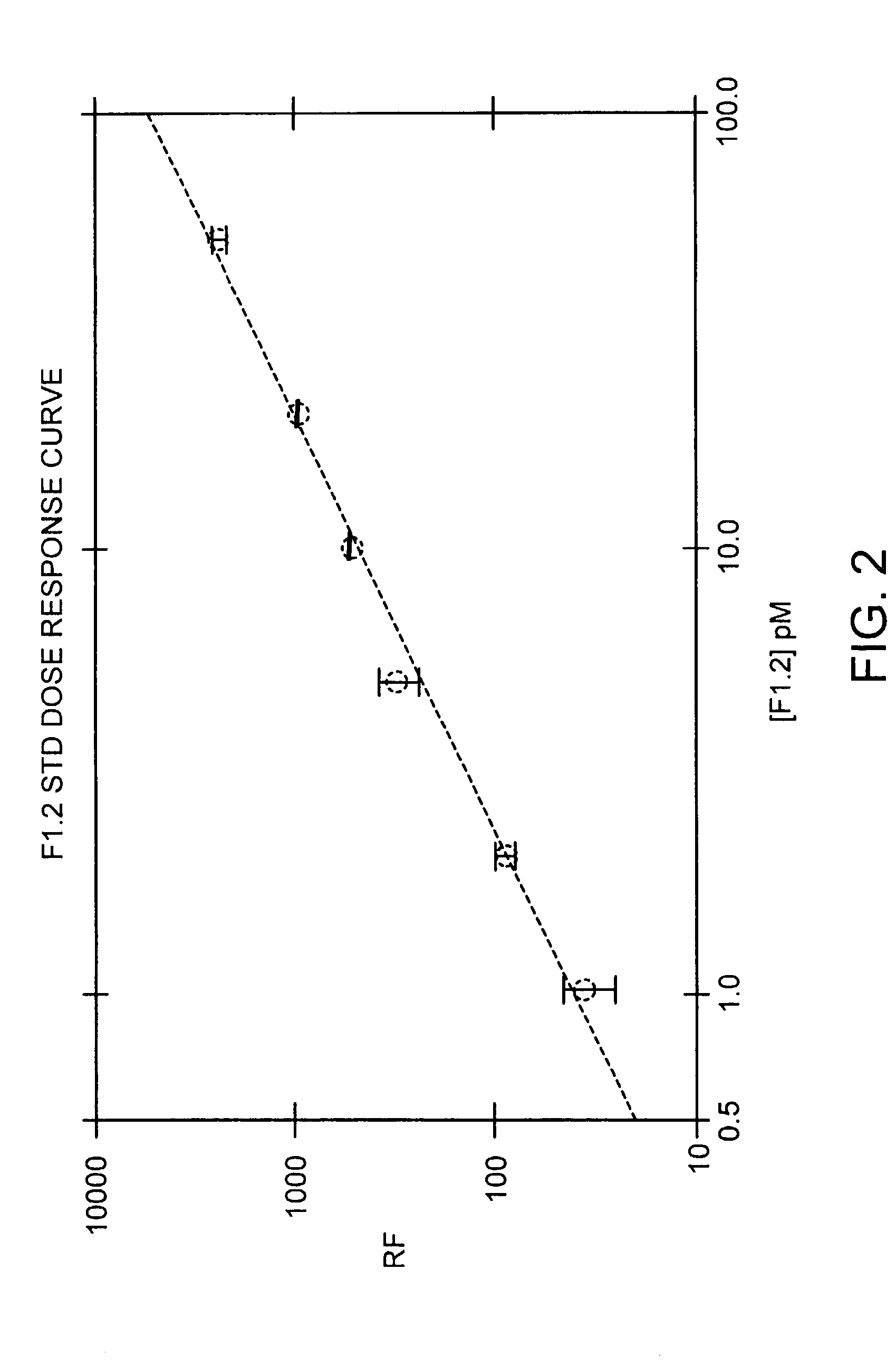
Immunoassay for F1.2 prothrombin fragment
a technology of prothrombin and immunoassay, which is applied in the field of immunoassay for f1.2 prothrombin fragment, can solve the problems of difficult quantification of f1.2 in plasma, and achieve the effect of convenient attachment poin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1b
Monoclonal Antibody CC1-5D
[0064] Monoclonal CC1-5d was derived from mice immunized with fragment F1.2. Mouse serum titration, cell fusion, hybridoma selection, ascites production and characterization were performed essentially as described in sections E through M above.
[0065] A. Affinity Data
[0066] The affinity constant for this monoclonal antibody was determined to be 7.04.times.10.sup.8 L / M. This constant was obtained using the method described in Friguet et al., Measurements of the True Affinity of Antigen-Antibody Complexes by Enzyme-Linked Immunoadsorbent Assay, 77, J. Immunol. Methods 305 (1985).
example 2
Rabbit Polyclonal Antibodies
[0067] A. Rabbit Polyclonal Antibody Preparation
[0068] Peptides were synthesized according to the procedure disclosed in Example 1A, Section A. Ovalbumin conjugates with peptide C700H were made according to Example 1A, Section C.
[0069] B. Rabbit Immunization Schedule
[0070] Rabbits were immunized by a series of sub Q injections in either Complete or Incomplete Freund's Adjuvant. Immunization spanned 38 days; at the end of this period, antiserum titers were checked.
[0071] Antisera from rabbits AB51-AB55 were tested serially at dilutions between 1:1000 to 1:64,000. Antibody dilutions were incubated for one hour at room temperature on microtiter plates coated with a variety of synthetic peptides or F1.2. The wells were washed three times with PBS containing 0.05% Tween 20 and incubated with goat anti-rabbit immunoglobulin / peroxidase for 1 hour. The wells were again washed three times as before and peroxidase was added for 15-30 minutes.
[0072] Rabbit antisera ...
example 3
Sandwich Assay Using Monoclonal Antibodies
[0073] Monoclonal TA1-5e is immobilized on a solid carrier, such as in microtiter wells. A sample of patient plasma containing F1.2 and other plasma proteins is then contacted with immobilized TA1-5e for a specified time and temperature (i.e. 2 hours at room temperature). At this stage insoluble complex is formed between immobilized TA1-5e and F1.2; any unreacted F1.2 is removed by washing the microwell.
[0074] A measured amount of monoclonal CC1-5d Fab fragment, covalently labelled with an enzyme such as alkaline phosphatase, is then added to the above insoluble complex and incubated for a specified time and temperature (i.e. 1 hour at room temperature). The unreacted portion of labelled CC1-5d is again removed by washing. The amount of bound, labelled antibody is determined using an alkaline phosphatase substrate, such as 4-methyl-umbelliferyll-phosphate.
[0075] The amount of bound, labelled antibody is directly proportional to the concentra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com