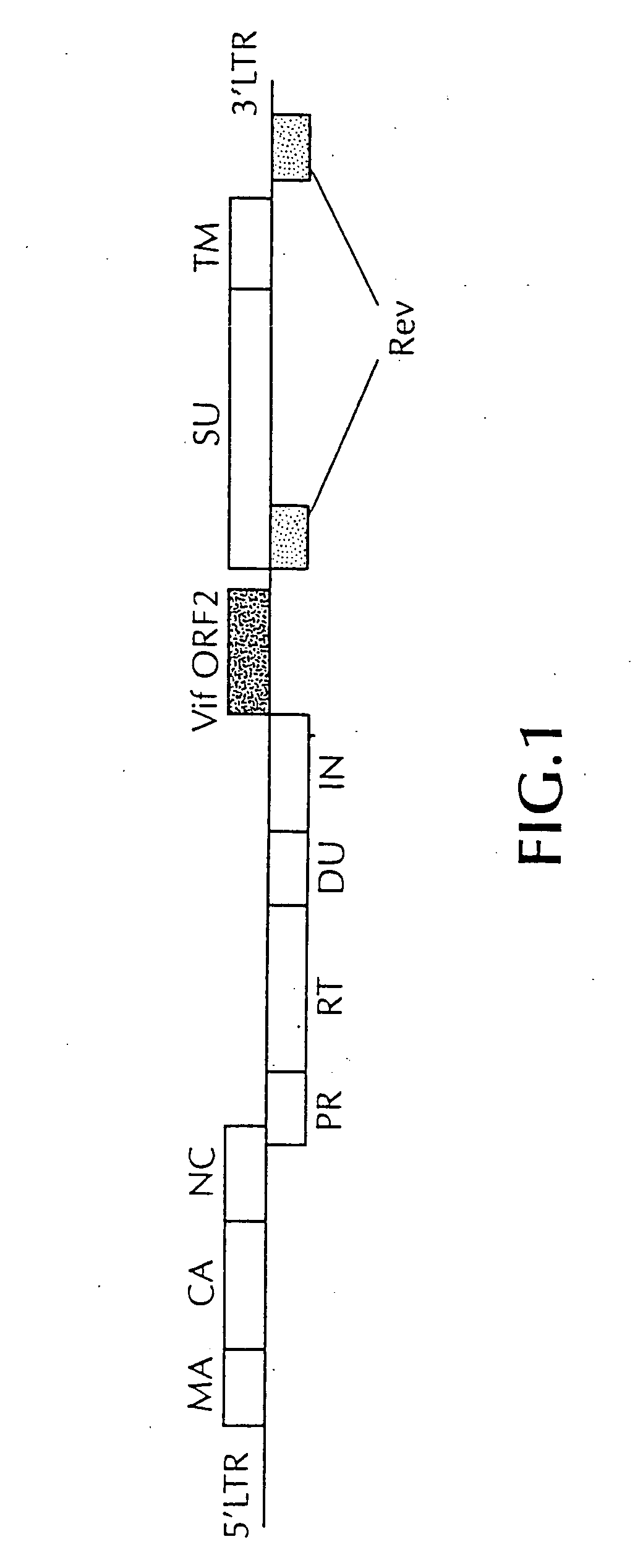
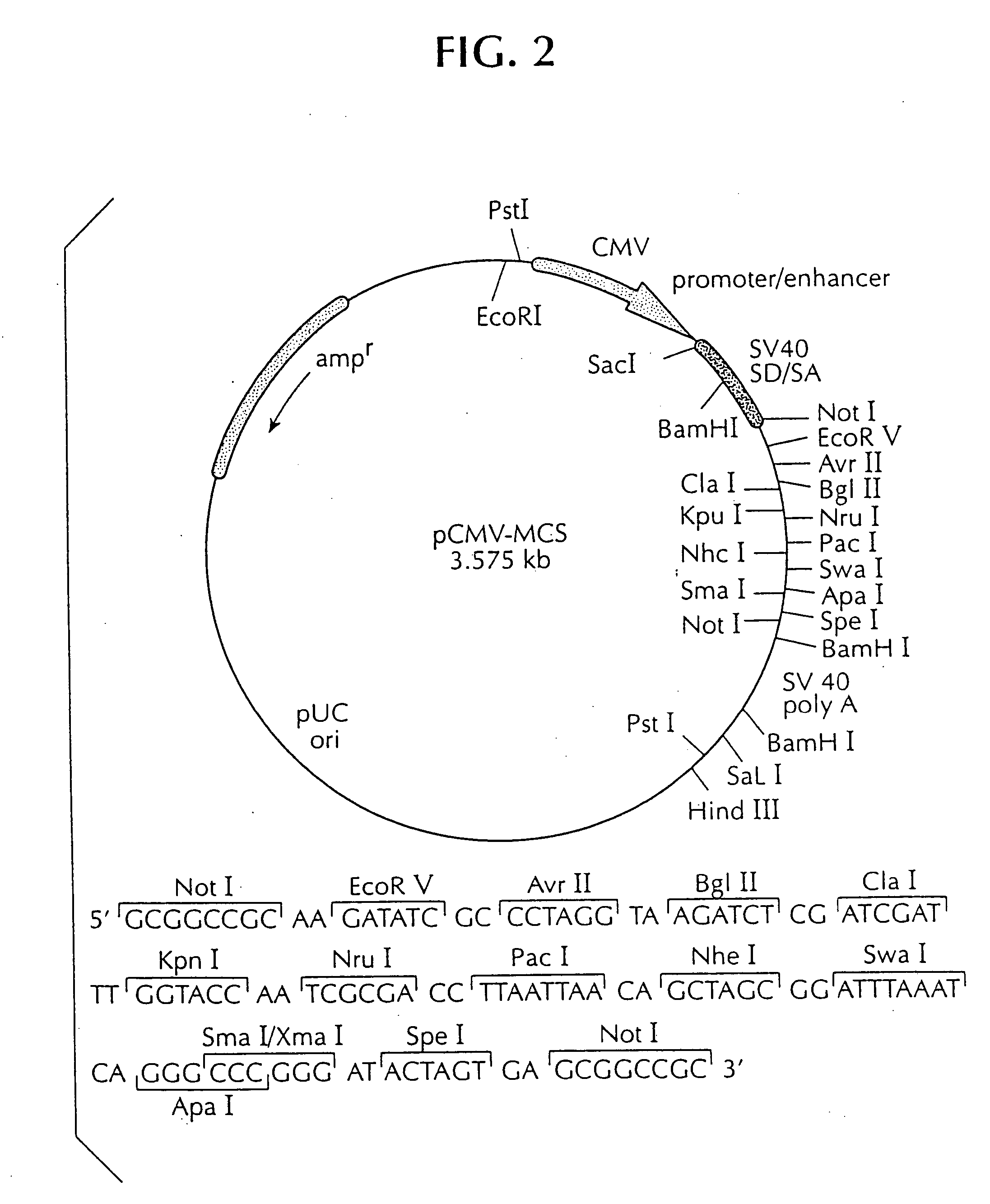
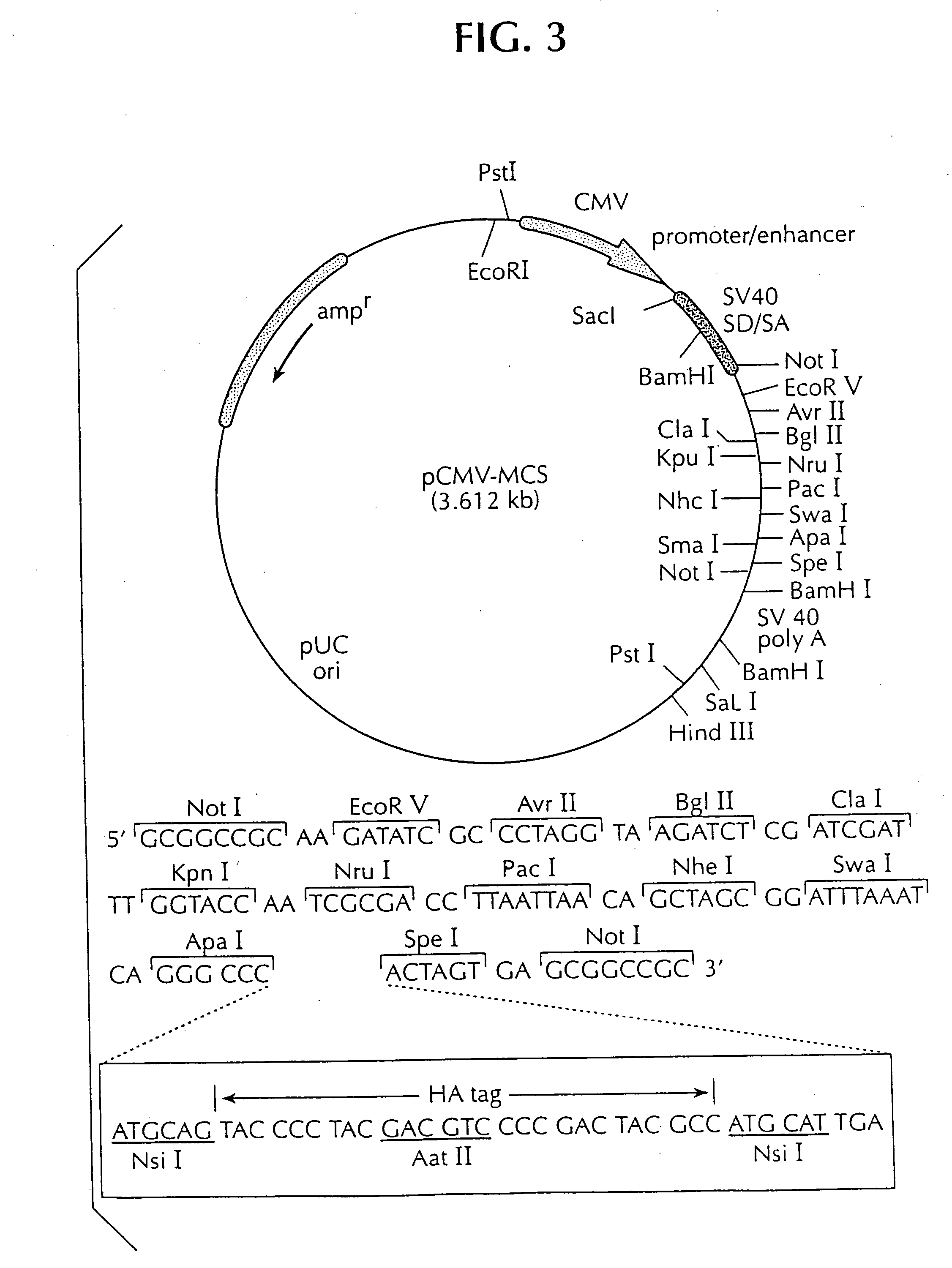
DNA vaccine against feline immunodeficiency virus
a technology of immunodeficiency virus and dna vaccine, applied in the field of animal health, can solve the problems of complex development of effective fiv vaccine, inability to protect against homologous challenge, and inability to fully understand the mechanism by which antibodies enhance fiv infection, so as to facilitate cellular uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
5.1. DNA Vaccin
5.1.1. Polynucleotide M I cul s
[0048] As used herein, the terms "DNA", "RNA", "gene," "polynucleotide molecule," "nucleotide sequence," "coding sequence," and "coding region" are intended to include both DNA and RNA polynucleotide molecules, and to refer to both single-stranded and double-stranded polynucleotide molecules. Thus, the term "DNA vaccine", as used herein, encompasses vaccines comprising either DNA or RNA, or both. Also, as used herein, the terms "gene," "coding sequence," and "coding region" are intended to refer to polynucleotide molecules that can be transcribed and translated (DNA), or translated (RNA), into an FIV structural or non-structural protein in a cat or in an appropriate in vitro host cell expression system when placed in operative association with appropriate regulatory elements. Polynucleotide molecules of the vaccine composition can include, but are not limited to, one or more prokaryotic sequences, eukaryotic sequences, cDNA sequences, ge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com