Delivery of compounds with rehydrated blood cells
a technology of rehydrated blood cells and compounds, applied in the field of fixed-dried blood cells, can solve the problems of limited use of cryopreserved platelets and normally liquid-stored for therapeutic delivery, limited practical application of cryopreserved platelets, and limited application of preserved blood cells in general
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples 1-5
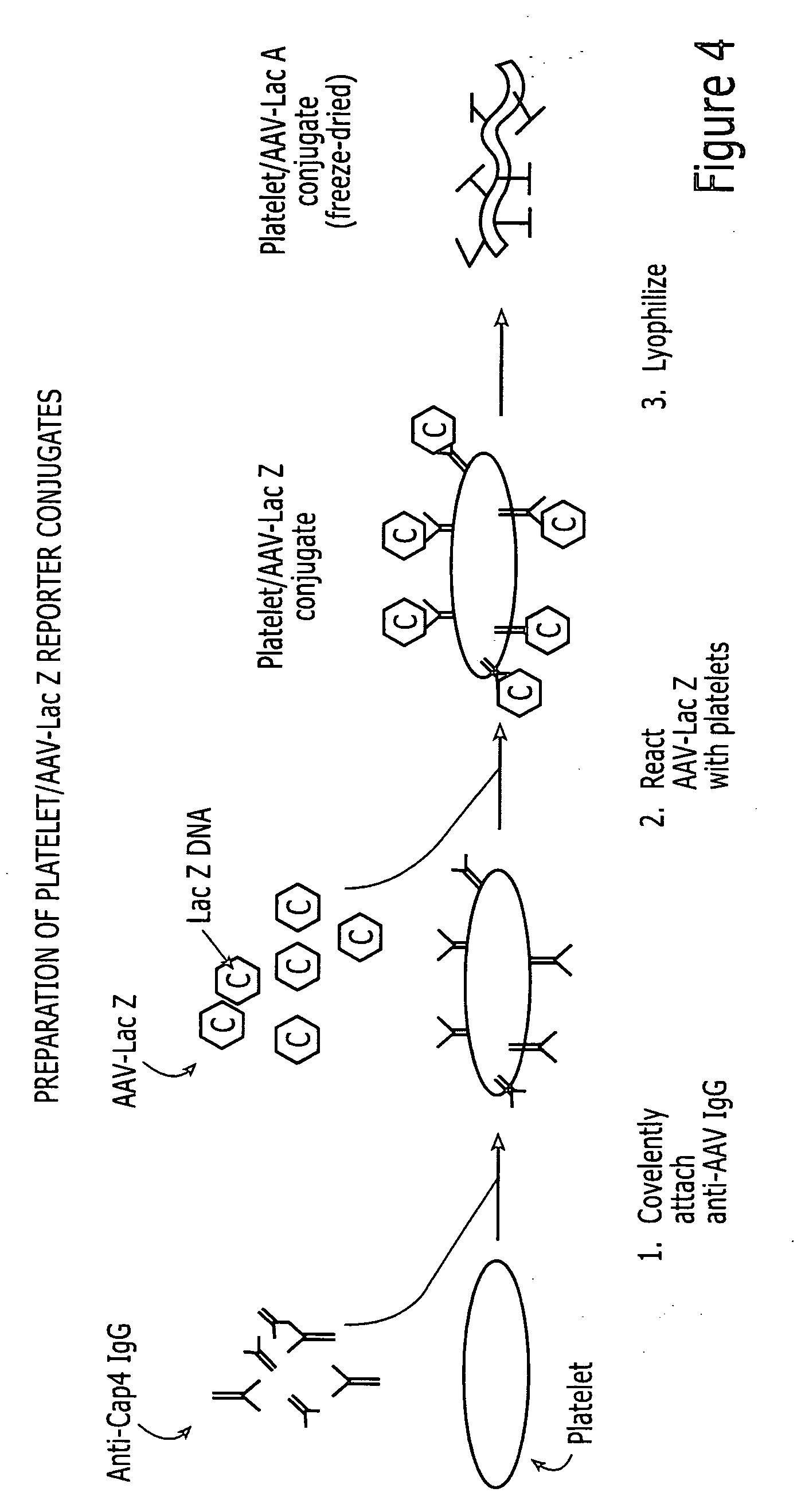
Attachment of Ribavirin to Platelets
[0067] These examples describe methods for the preparation and characterization of reconstituted platelets having ribavirin and rFVIIa coupled thereto.
example 1
Synthesis of Ribavirin-Polylysine Polymers
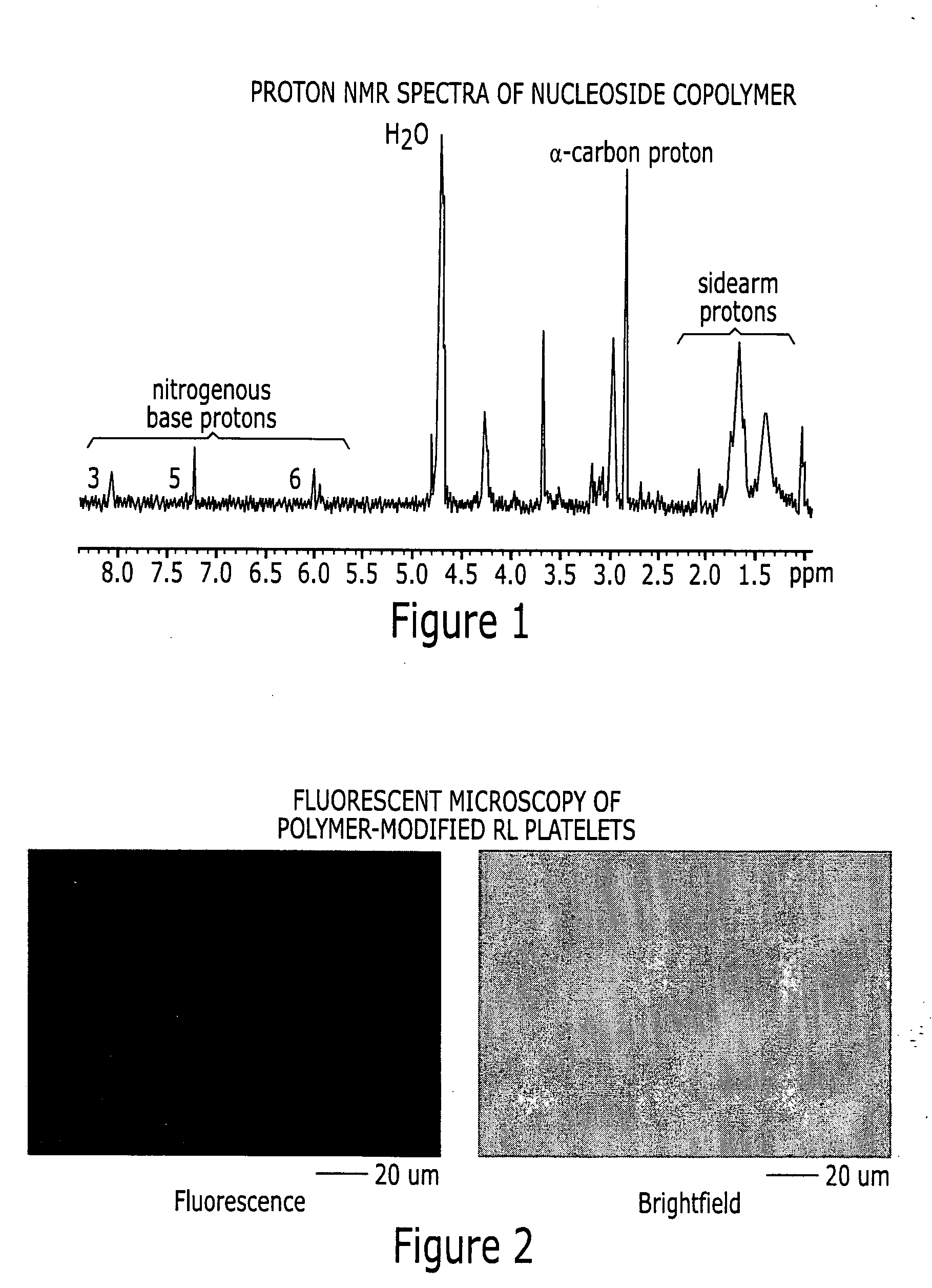
[0068] Ribavirin is chemically phosphorylated for ribavirin monophosphate (RMP) as detailed by Yoshikawa et al., Tetrahedron Lett. 50, 5065-5068 (1967)). Ribavirin monophosphate (RMP) is coupled to polylysine via a pH-sensitive phosphoramide linkage in accordance with the procedure of Di Stefano and Fiume (Trends in Glycosci. and Glycotech. 50, 461-472 (1997)) or a simplified procedure is based on the formation of an imidazole-ribavirin adduct (see Chu et al., Nuc. Acids. Res. 11, 6513-6529 (1983)). The conjugation chemistry for ribonucleosides polymer synthesis was tested with uracil rather than ribavirin. We do not anticipate that the difference in nitrogenous base structure between uracil and ribavirin will have a large effect on the synthesis.
[0069] Polylysine (=205 kDa for ˜1,400 residues lysine / molecule) was reacted with FITC (fluorescein isothiocyanate) and SANPAH (N-succinimidyl-6-[4′-azido-2′-nitrophenylamino] hexanoate) to respec...
example 2
Attachment of Ribavirin-Polylysine Polymers for Ribavirin-Loaded Lyophilized Platelets
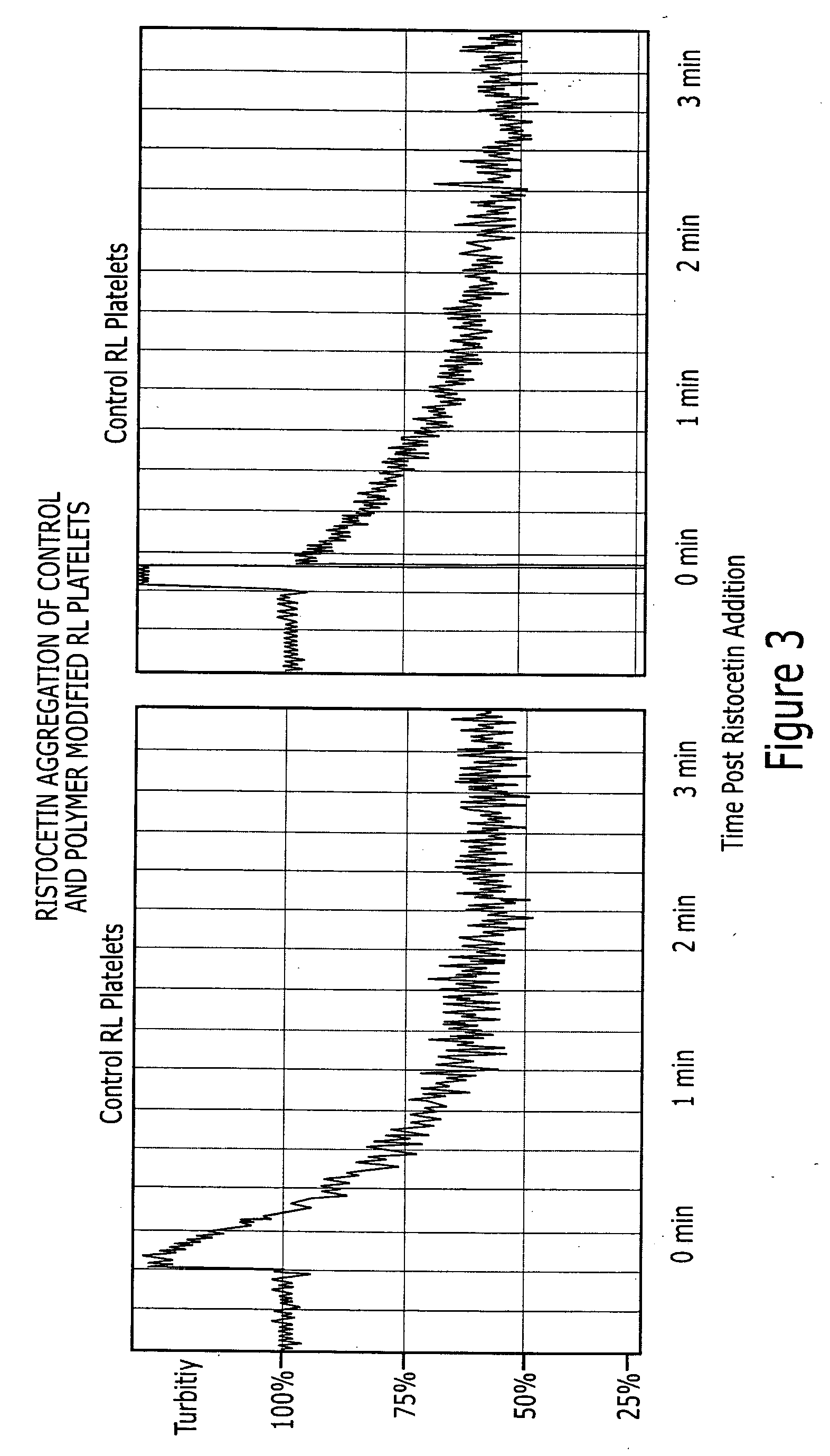
[0073] The procedure for preparing lyophilized platelets involves four steps: Removal of platelets from excess plasma proteins, mild paraformaldehyde cross-linking to stabilize cellular structures, removal of platelets from unreacted cross-linker and lyophilization (Read et al, U.S. Pat. No. 5,651,966). After removal of the excess paraformaldehyde, the platelets are mixed with varying concentrations of the ribavirin polymer and exposed to visible light to activate the SANPAH moieties for the formation of covalent linkage with the platelet surface. The platelets are then lyophilized with standard procedures. Platelets are prepared with different amounts of ribavirin by varying the concentration of ribavirin-copolymer in the coupling step. The ability of the ribonucleoside copolymer to covalently couple to RL platelets was studied by incubating the cells with the delivery polymer in visible-spectrum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com