Use for deferiprone
a deferiprone and prone technology, applied in the field of deferiprone, can solve the problems of dangerous iron overload, widespread iron overload in patients, toxic degenerative changes in liver, endocrine organs, etc., and achieve the effect of reducing iron stores in the hear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0117] Numerical Reference in this discussion is made to the list of references listed in the background of the invention.
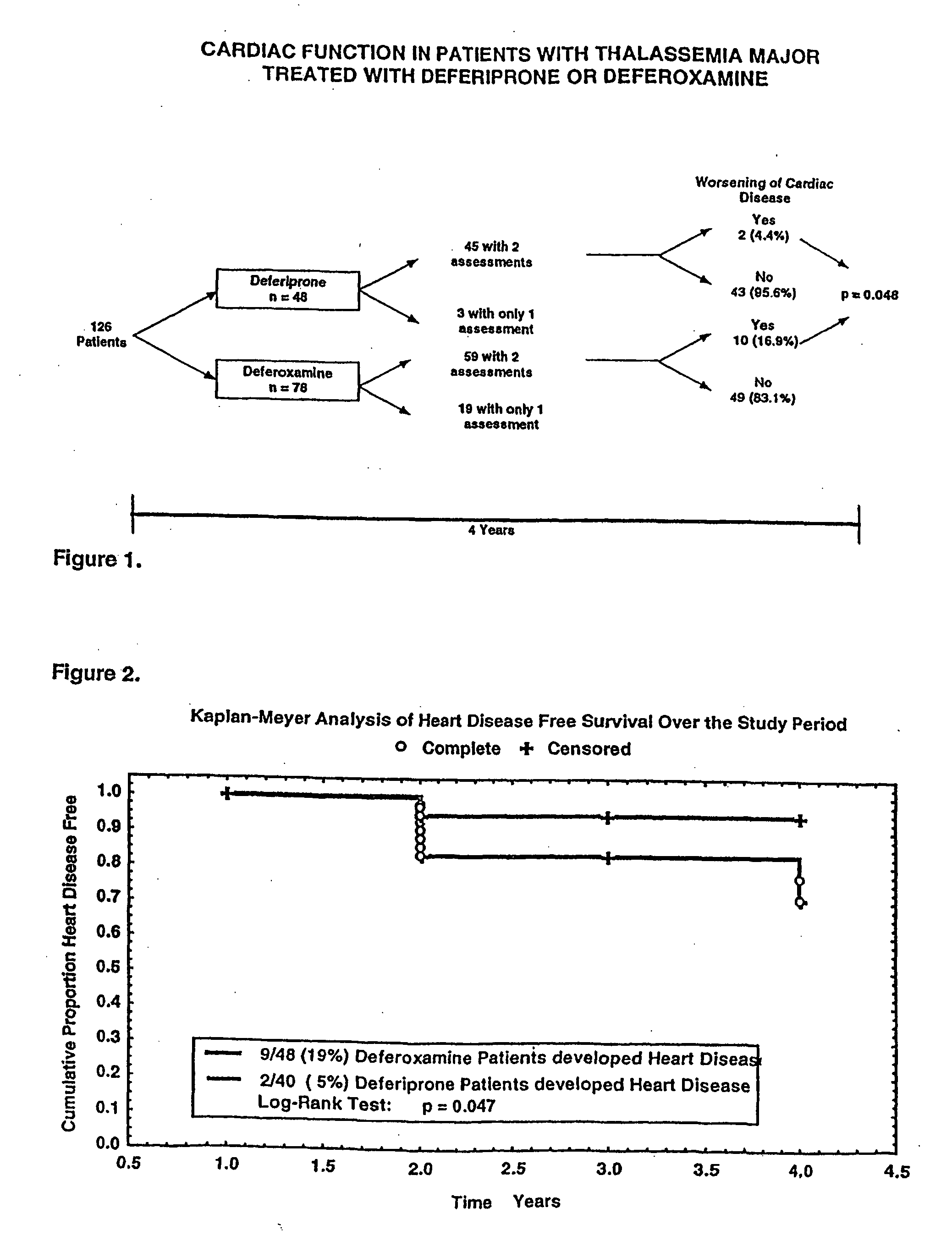
[0118] The efficacy of iron chelation by desferrioxamine therapy, in subjects with thalassemia major is known. Daily subcutaneous infusions of desferrioxamine has been shown to ameliorate hepatic, cardiac and endocrinological dysfunction, improve growth and sexual maturation, and prolong survival in iron-overloaded thalassemia major patients.1,2,3,4,5 However, iron-induced cardiac disease remains a frequent cause of morbidity in patients with thalassemia and is still responsible for 70% of the deaths among those subjects.6 A sustained reduction in iron load, as measured by the proportion of serum ferritin results below 2500 μg / L, and the ability to comply with daily infusions of desferrioxamine have been reported to be the most important factors in the survival among patients with thalassemia major.1,7 The age at the start of chelation therapy and the hepatic ir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com