Methods of treating tumors
a tumor and tumor technology, applied in the field of cancer therapy, can solve the problems of high mortality rate, low specificity, chemotherapeutic agents, etc., and achieve the effects of reducing toxicity, prolonging clinical half-life, and improving ability to cross the blood-brain barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cytotoxic and Mechanistic Evaluation of Antineoplastic Agents
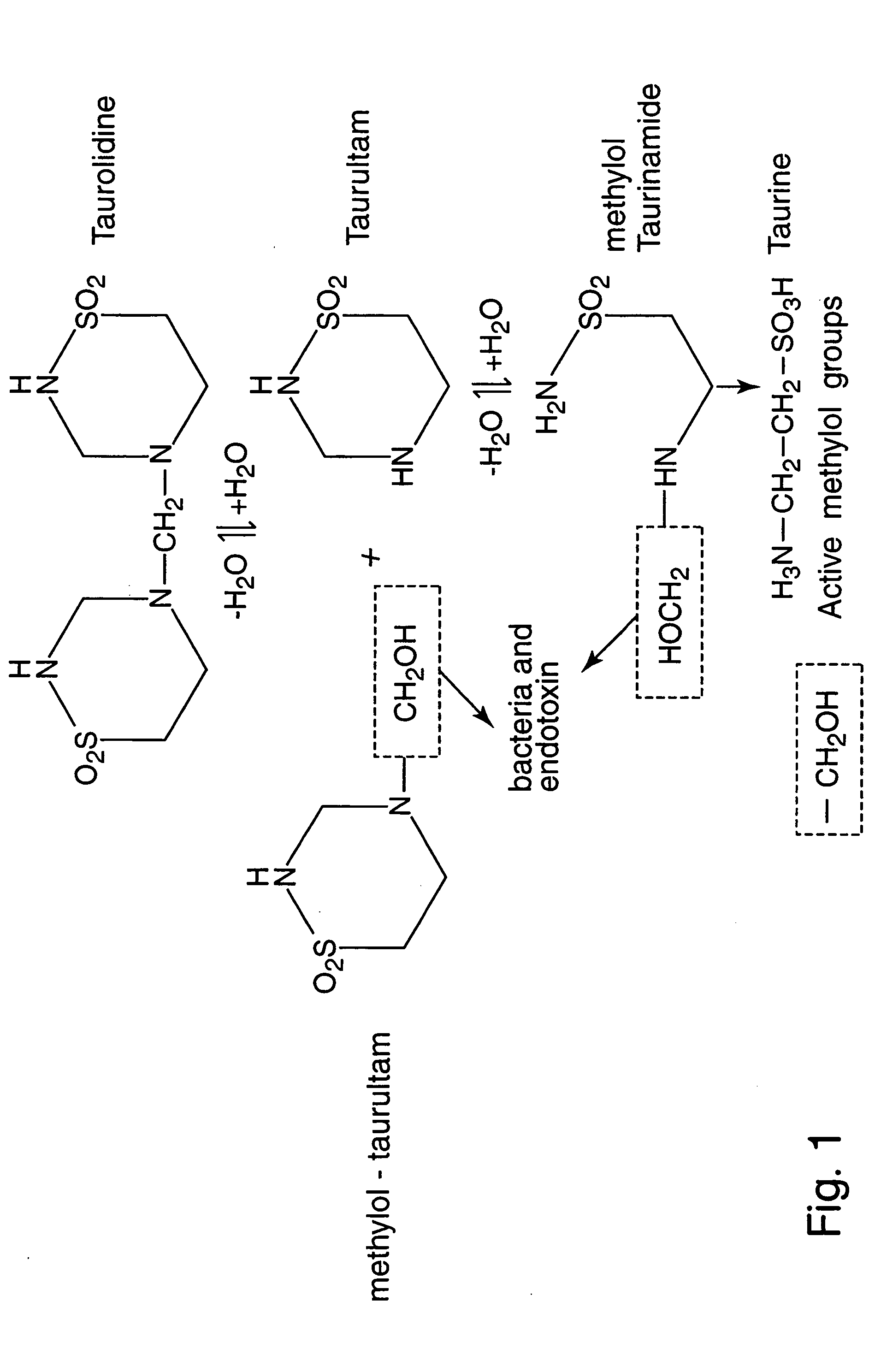
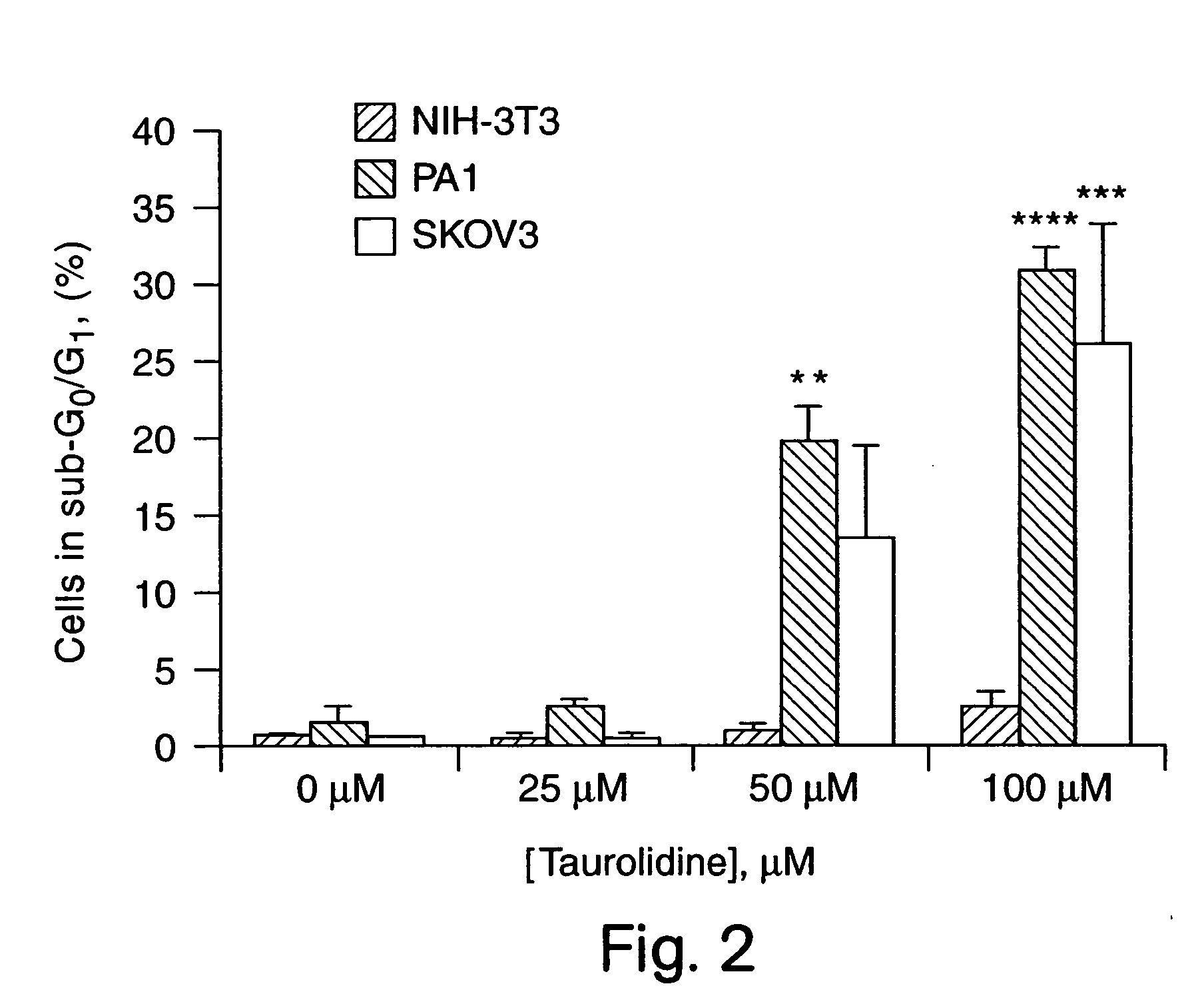
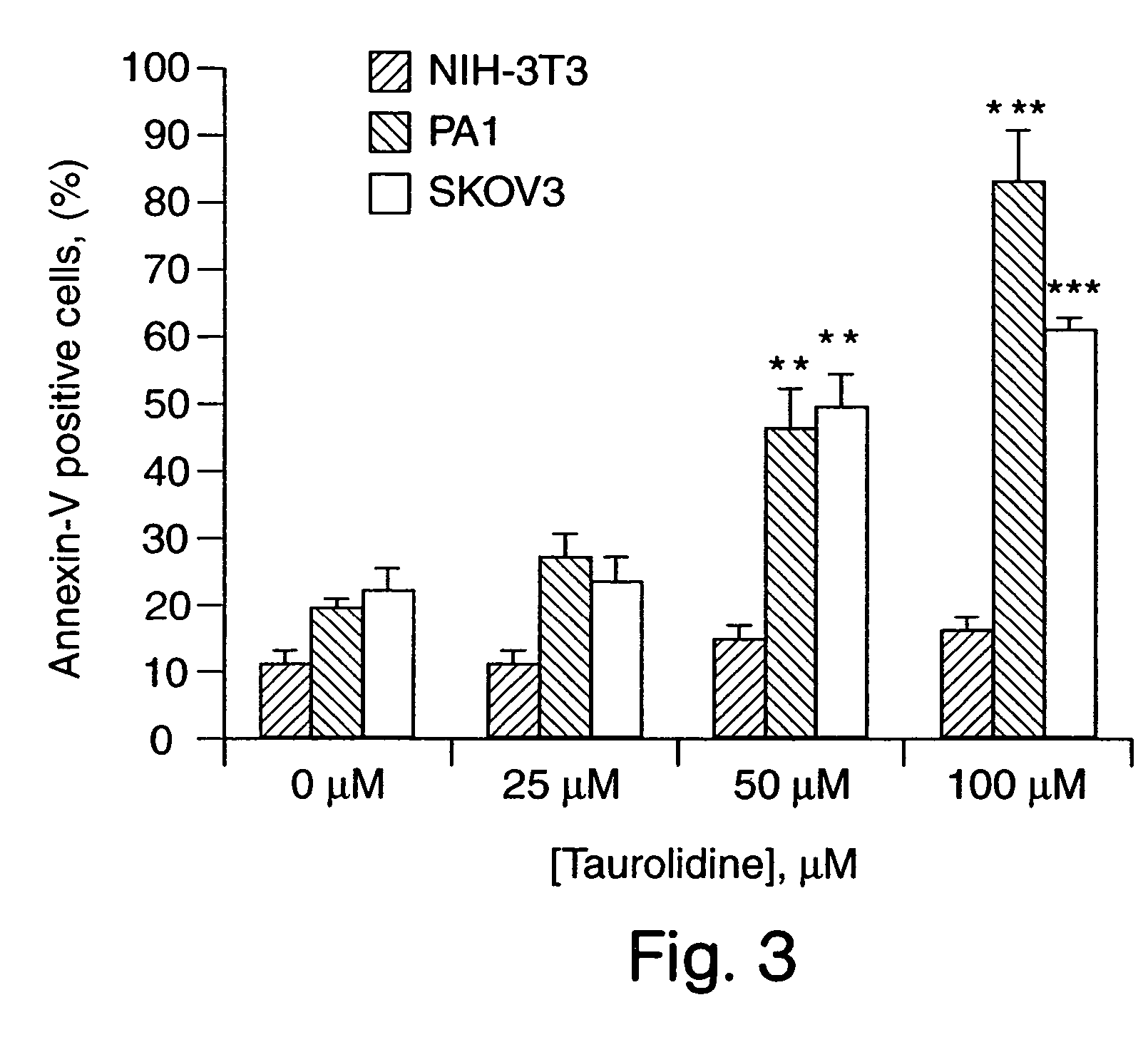
[0055] Taurolidine was found to be active at inhibiting the growth of a variety of human tumor cell lines in vitro. PA-1 and SKOV-3 human ovarian tumor cell lines and NIH-3T3 murine fibroblasts were used to determine the mechanism of antitumor activity. The studies revealed that this effect was associated with alterations in DNA structure, cell membrane components, and protein cleavage that were consistent with the induction of apoptosis specifically in tumor cells. Antineoplastic evaluation of Taurolidine in nude mice bearing intraperitoneal xenografts of human ovarian tumors demonstrated that this agent significantly inhibited tumor development and growth in vivo.
[0056] To study neoplastic activity, Taurolidine was formulated as 2% solution in 5% Kollidon 17PF. Standard cell culture growth media (e.g., High glucose DMEM, RPMI 1640, McCoy's 5A, and F12K), trypsin, and fetal bovine serum (FBS) were all purchased from GIB...
example 2
Clinical Use
[0083] Taurolidine was administered by i.p. lavage immediately following surgery for removal of recurrent ovarian tumors. For patients with glioblastoma, Taurolidine was administered systemically. To date, Taurolidine has been well tolerated in these patients.
[0084] Four patients, which were diagnosed with advanced recurrent glioblastoma multiforma, were treated with taurolidine. The prognosis for this group of patients was determined to be approximately 8 weeks of survival. Each patient received at least one 5 week regimen in which 20 g of taurolidine was infused intravenously into the arm over a period of 6 hours twice a week. In 3 out of the 4 patients treated, the tumor mass decreased or stayed the same; and in one case, a slight increase was seen. At 14 weeks after the initiation of therapy, each of the patients remains alive, having exceeded the 8 week prognosis. A beneficial clinical effect was achieved in these brain tumor patients with systemic administration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com