Nitrosated and nitrosylated heme proteins
a technology of heme proteins and nitrosylated heme, which is applied in the direction of peptide/protein ingredients, peptide sources, drug compositions, etc., can solve the problems of life-threatening diseases, oxygen-hemoglobin binding, oxygen transport, etc., and achieve the effect of inhibiting platelet function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
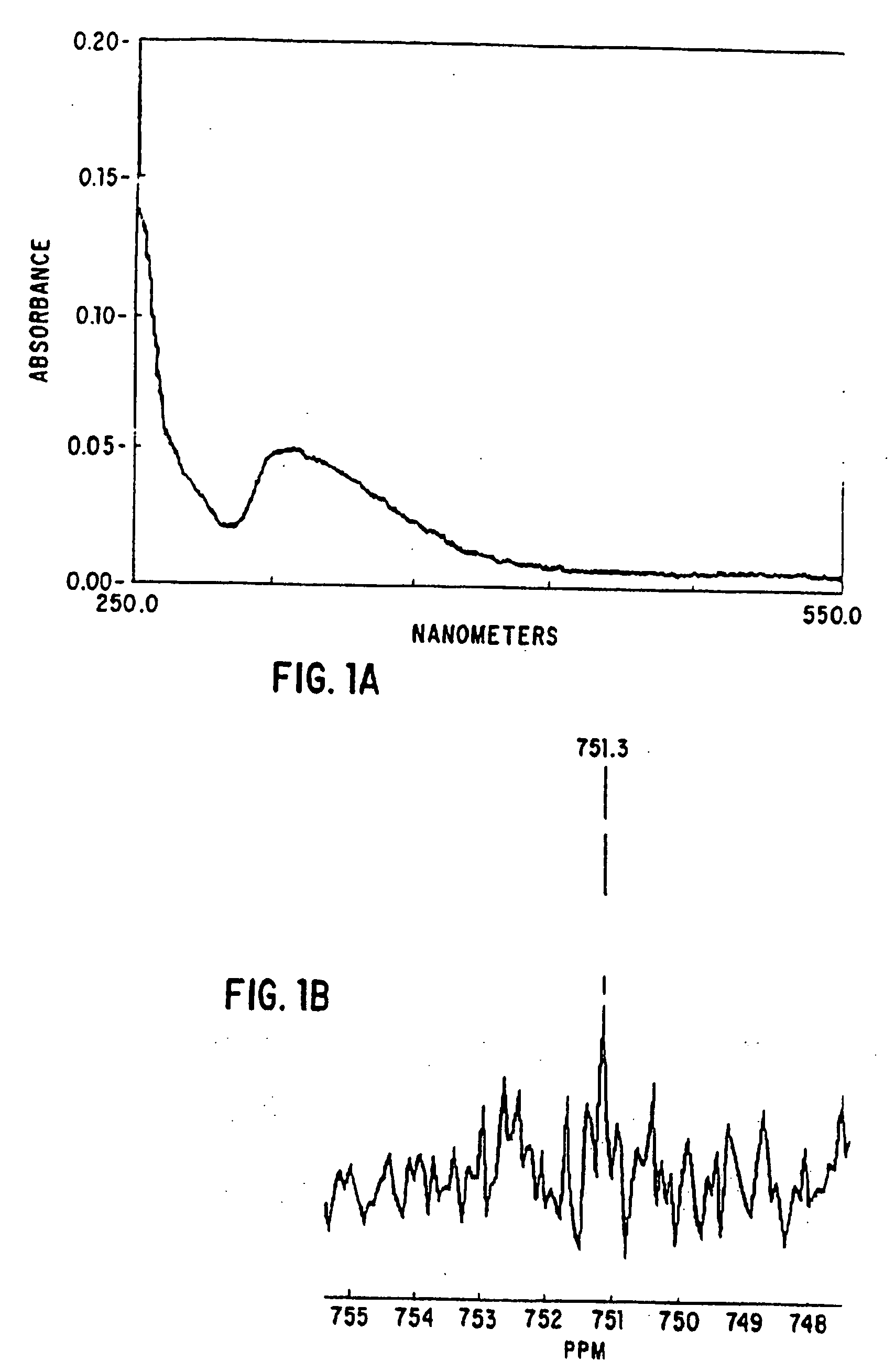
Synthesis of S-Nitroso-t-PA
[0151] A. Nitrosylation of t-PA [0152] 1. Materials
[0153] t-PA was kindly provided by Genentech, Inc. San Francisco, Calif. Reactivated purified plasminogen activator inhibitor-1 (PAI- 1) and a panel of six murine anti-t-PA monoclonal antibodies were kindly provided by Dr. Douglas E. Vaughan. Horse-Radish Peroxidase linked-sheep antimurine antibodies were purchased from Amersham Corp., Arlington, Ill. Sodium nitrite was purchased from Fisher Scientific, Fairlawn, N.J. H-D-isoleucyl-L-prolyl-L-arginyl-p-nitroanilide (S2288) and H-D-valyl-L-leucyl-L-lysyl-p-nitroanilide (S2251) were purchased from Kabi Vitrum, Stockholm, Sweden. Human fibrinogen purified of plasminogen and von Willebrand factor, was obtained from Enzyme Research Laboratories, South Bend, Ind. Epinephrine, ADP and iodoacetamide were purchased from Sigma Chemical Co., St. Louis, Mo. Bovine thrombin was obtained from ICN, ImmunoBiologicals (Lisle, Ill.) Radioimmunoassay kits for the determina...
example 2
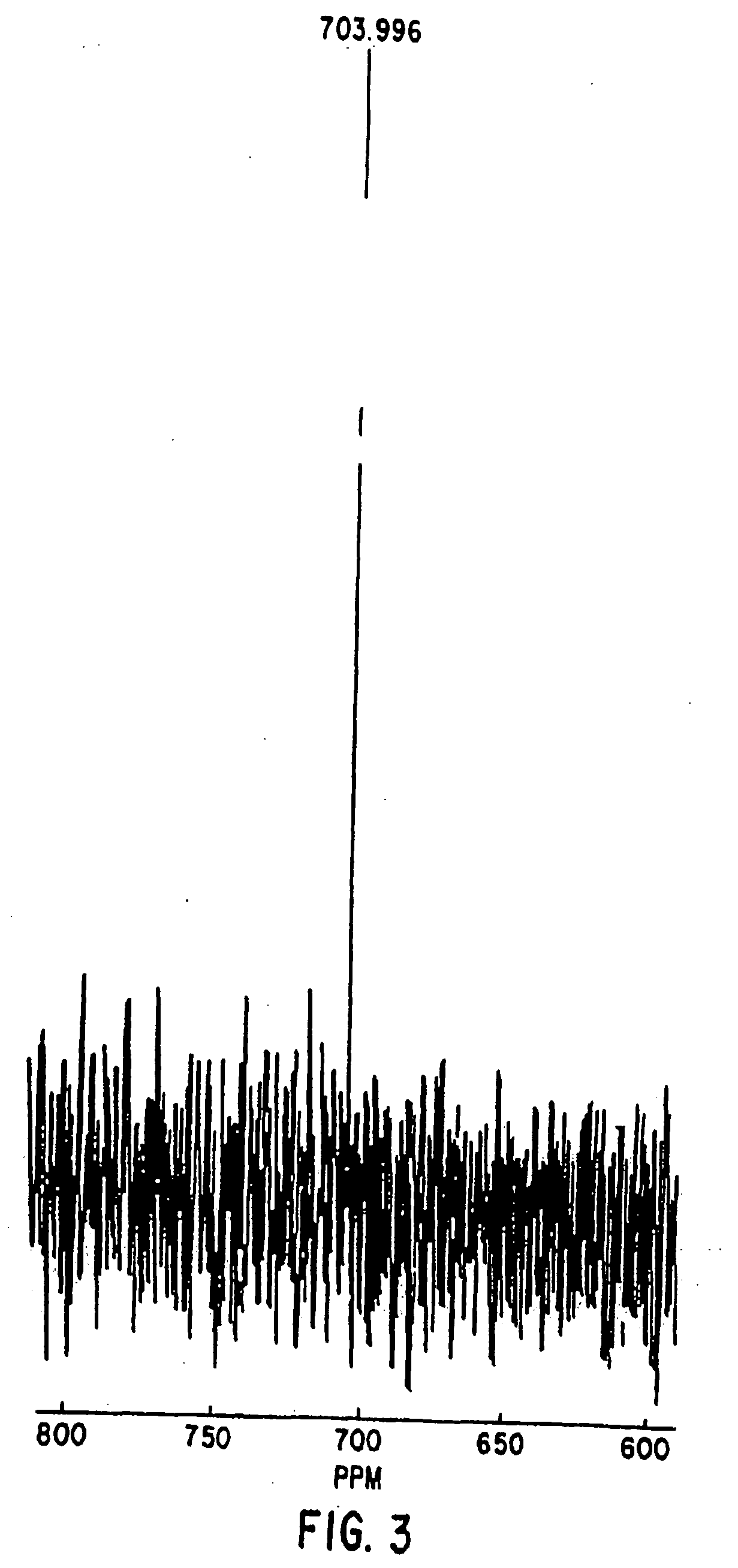
Synthesis of S-Nitroso-BSA
[0174] A. Nitrosylation
[0175] In the first method, nitrosylation of BSA was accomplished by incubating BSA (200 mg / ml with NO generated from equimolar NaNO2 in 0.5N HCl (acidified NaNO2) for thirty minutes at room temperature. Solutions were titrated to pH 7.4 with equal volumes of 1.0 N NaOH and Tris Buffered Saline (TBS), pH 7.4 0.05 M L-arginine. Dilutions were then made as necessary in TBS.
[0176] In the second method, nitrosylation was achieved in helium-deoxygenated solutions of 0.1 M sodium phosphate (pH 7.4) by exposing the protein solution in dialysis tubing to authentic NO gas bubbled into the dialysate for fifteen minutes. The proteins were then dialyzed against a large excess of 0.01 M phosphate buffer at pH 7.4 to remove excess oxides of nitrogen.
[0177] In the third method, proteins were incubated with bovine aortic endothelial cells stimulated by exposure to high shear forces to secrete EDRF, as in Example 1(A). As a corollary of this metho...
example 3
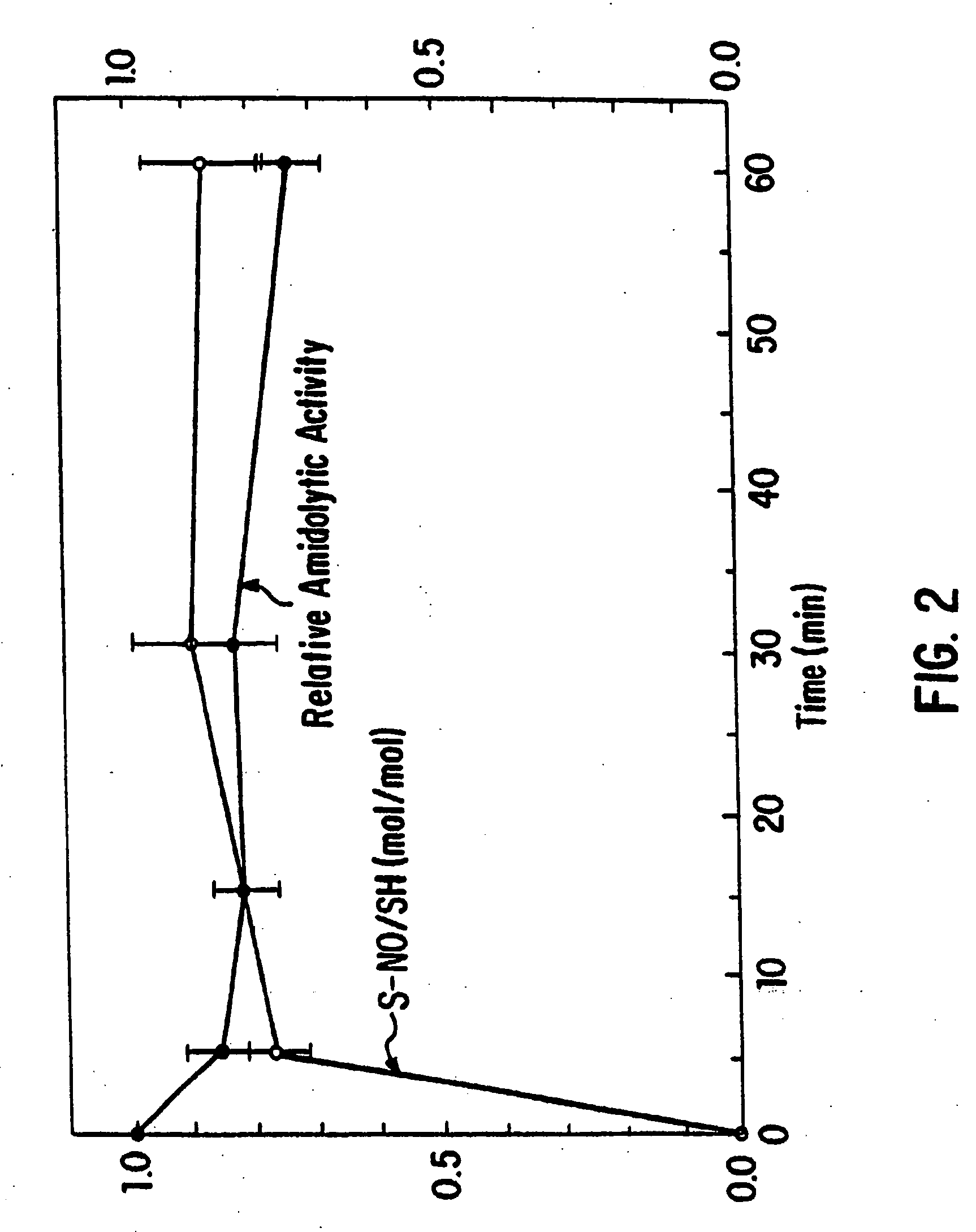
Synthesis of S-Nitroso-Cathepsin B
[0182] Nitrosylation of cathepsin, and determination of S-nitrosothiol formation, was accomplished according to the methods described in Example 2. The stoichiometry of S-nitrosothiol / protein molecules for cathepsin is shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com