Methods and compounds for prevention and treatment of elevated intraocular pressure and related conditions
a technology of elevated intraocular pressure and compound, which is applied in the direction of drug compositions, peptides, cardiovascular disorders, etc., can solve the problems of peripheral vision loss and sometimes central vision loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Cloning and Characterization of the Nucleic Acid Encoding R-14
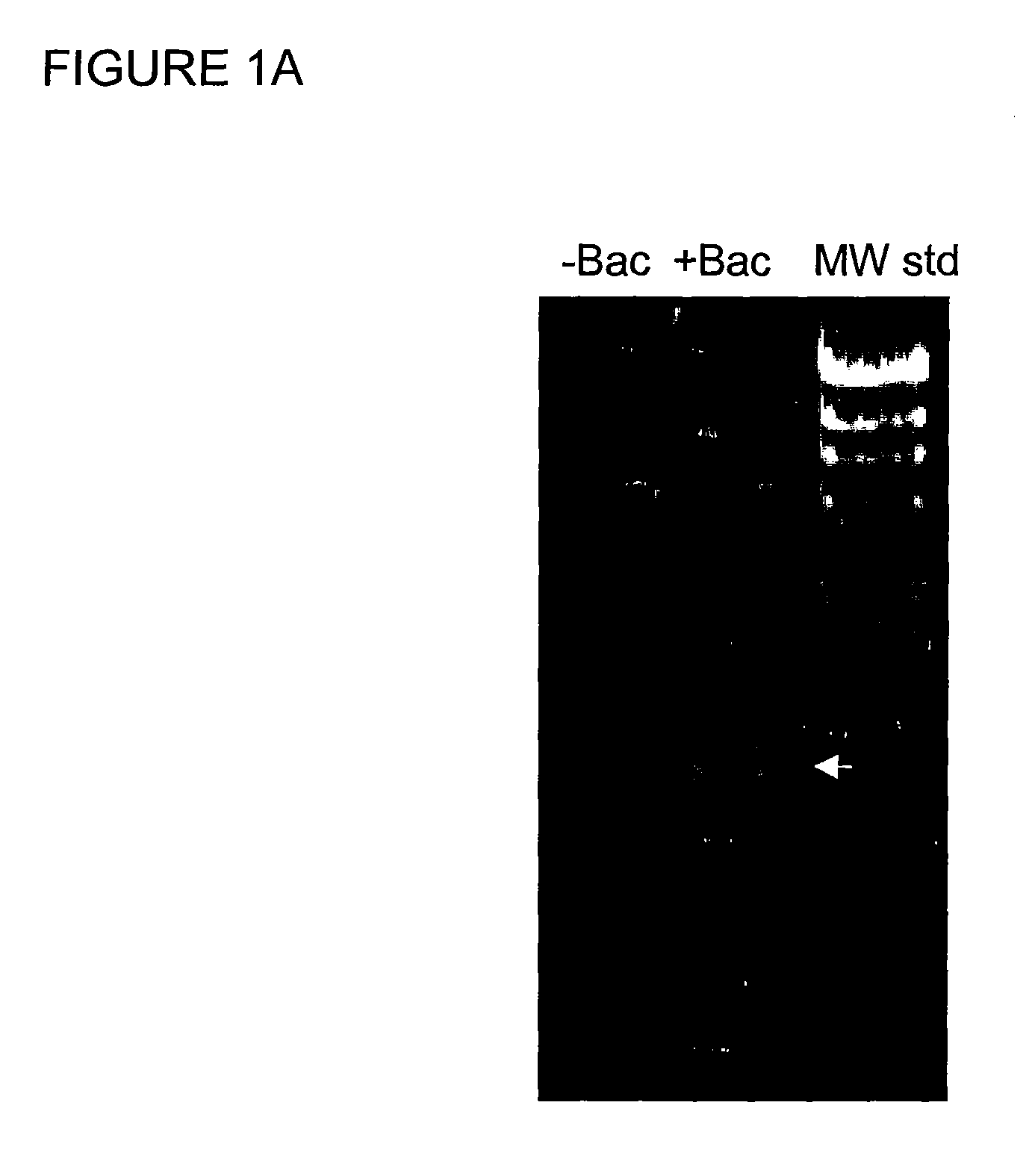
[0198] In silico analysis of EST # HTM1-025F1 (GenBank Acc.: BE439409) against human genome using BLAST-N (v. 2.2.1) program resulted in a hit of 99.8% similarity to HTM1-025F1 in contig NT—009307.3, AC020568.4 on chromosome 11. A 966 bp fragment was amplified from a BAC clone, RP11-206Cl obtained from the Sanger Center, UK, by PCR, using gene specific primers, 5′GATTCAACCATCCCAGTCTTGGGTACAG3′ (SEQ ID NO: 6) and 5′TTACTGCTCCAATCTGCTTCCCGACAGC3′ (SEQ ID NO: 7). PCR was done using Taq HiFi (Invitrogen, Calif.) following the protocol suggested by the manufacturer (annealing at 60° C. for 30 sec, elongation at 68° C. for 80 sec: total cycles-35). The PCR products were separated using 1% agarose gels. A photograph of the gel is shown in FIG. 1(A). −Bac: No plasmid DNA; +Bac: contains plasmid DNA (0.1 μg); MW std: λ Hind III digest. The ˜960 bp fragment (indicated by arrow) was cloned into pcDNA4HisMax TOPO TA (Invitrogen, Cal...
example 2
Expression of R-14 mRNA in Human Trabecular Meshwork Tissue
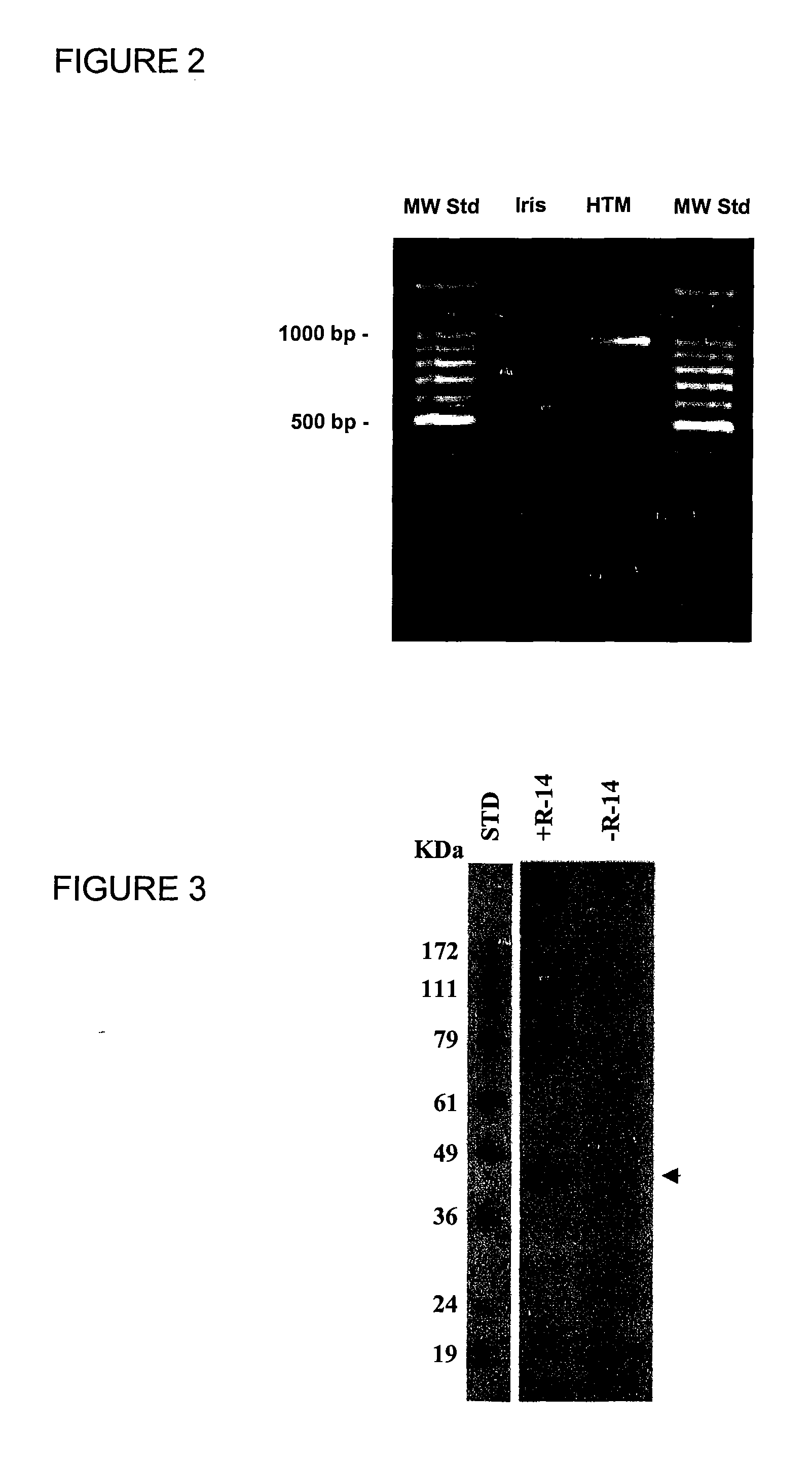
[0201] Method: RT-PCR of total mRNA isolated from human trabecular meshwork (HTM) and human iris (HI) tissues. Aliquots of total RNA (1 μg) were reverse transcribed (50° C. for 30 min) and the reaction mixture was amplified for 35 cycles (60° C. for 30 sec) using gene-specific primers SuperScript One-Step RT-PCR (InVitrogen). Resolution of the PCR products by agarose gel electrophoresis showed a single DNA fragment of 1 Kb from HTM tissue, but not from iris tissue. Sequencing of the DNA fragment from HTM tissue identified the 1 Kb fragment to contain the R-14 reading frame.
example 3
Expression of Cloned Human R-14 Receptor in Human Cells
[0202] Cell culture: HEK293 cells were grown in complete DMEM (10% fetal calf serum, 0.1% Penicillin and streptomycin, 2 mM glutamine, 0.5ug / ml fungizone, 5ug / ml gentamicin) until −70% confluent. Cells were transfected using Lipofectamine 2000 (InVitrogen) according to the manufacturer's recommendations using 12.5 μg DNA and 25 μl Lipofectamine in DMEM. Media was replaced after 48 hours for complete DMEM (The cells were processed immediately to detect transient expression of R-14), supplemented with 500 μg / ml Zeocin. After 3 weeks in culture, the zeocin-resistant cells (R14 / 293) were split and cultured in complete DMEM with 100 μg / ml Zeocin.
[0203] Immunoblotting of R-14 proteins: Confluent cells were lysed in TNE (10 mM Tris-HCl pH 7.4, 0.1 mM EDTA, 0.85% NaCl, 1% NP-40) buffer containing protease inhibitor cocktail. Aliquots (100 μg protein) of supernatants were denatured in 50 μl of SDS-loading buffer by boiling for 5 minute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal melting point | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
| body temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com