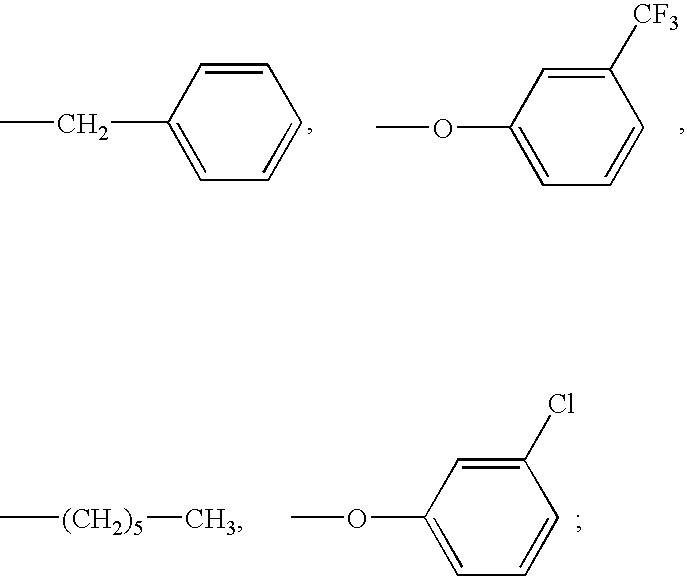
Patents
Literature
551 results about "Glaucoma present" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
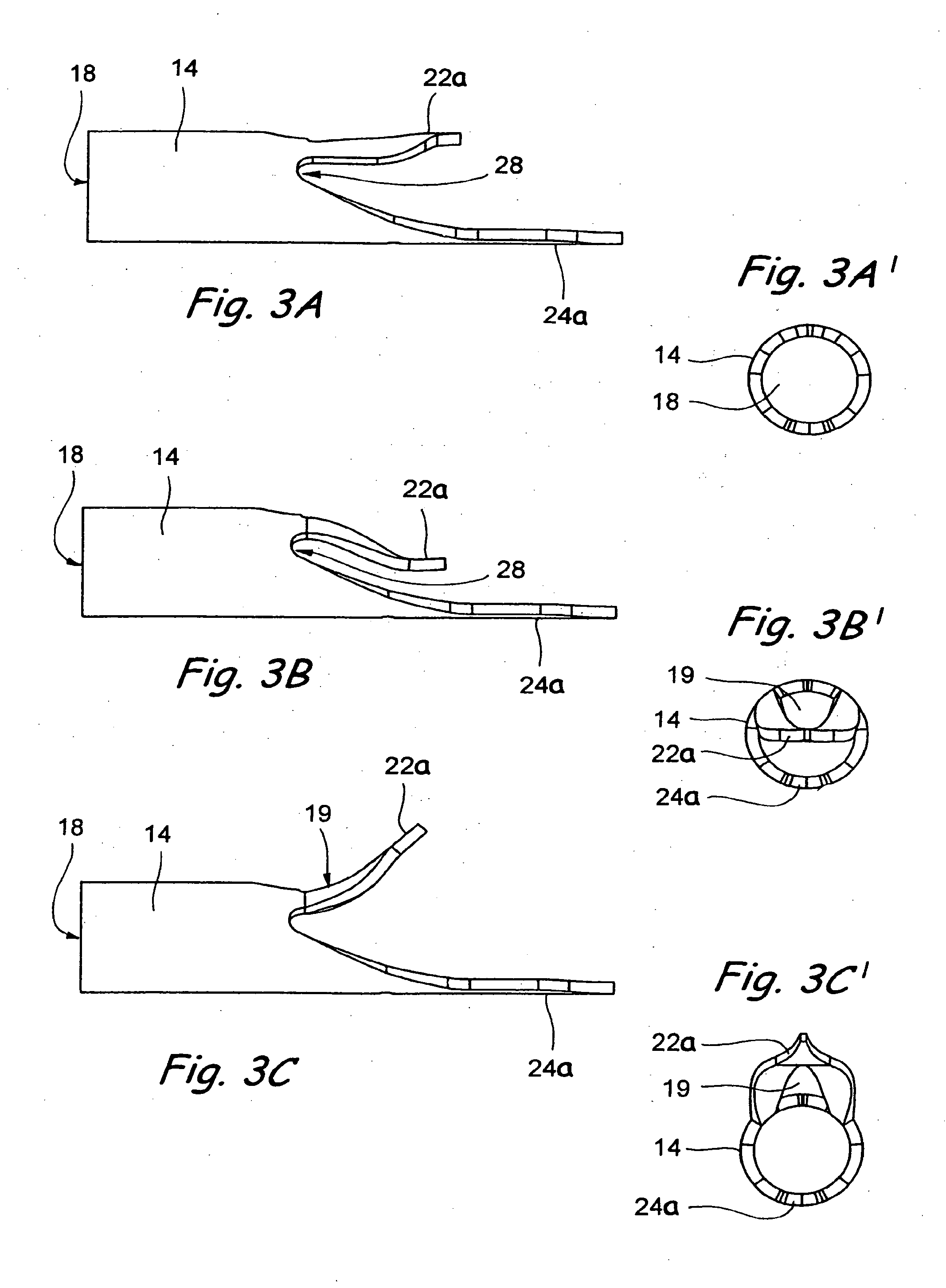
Expandable glaucoma implant and methods of use
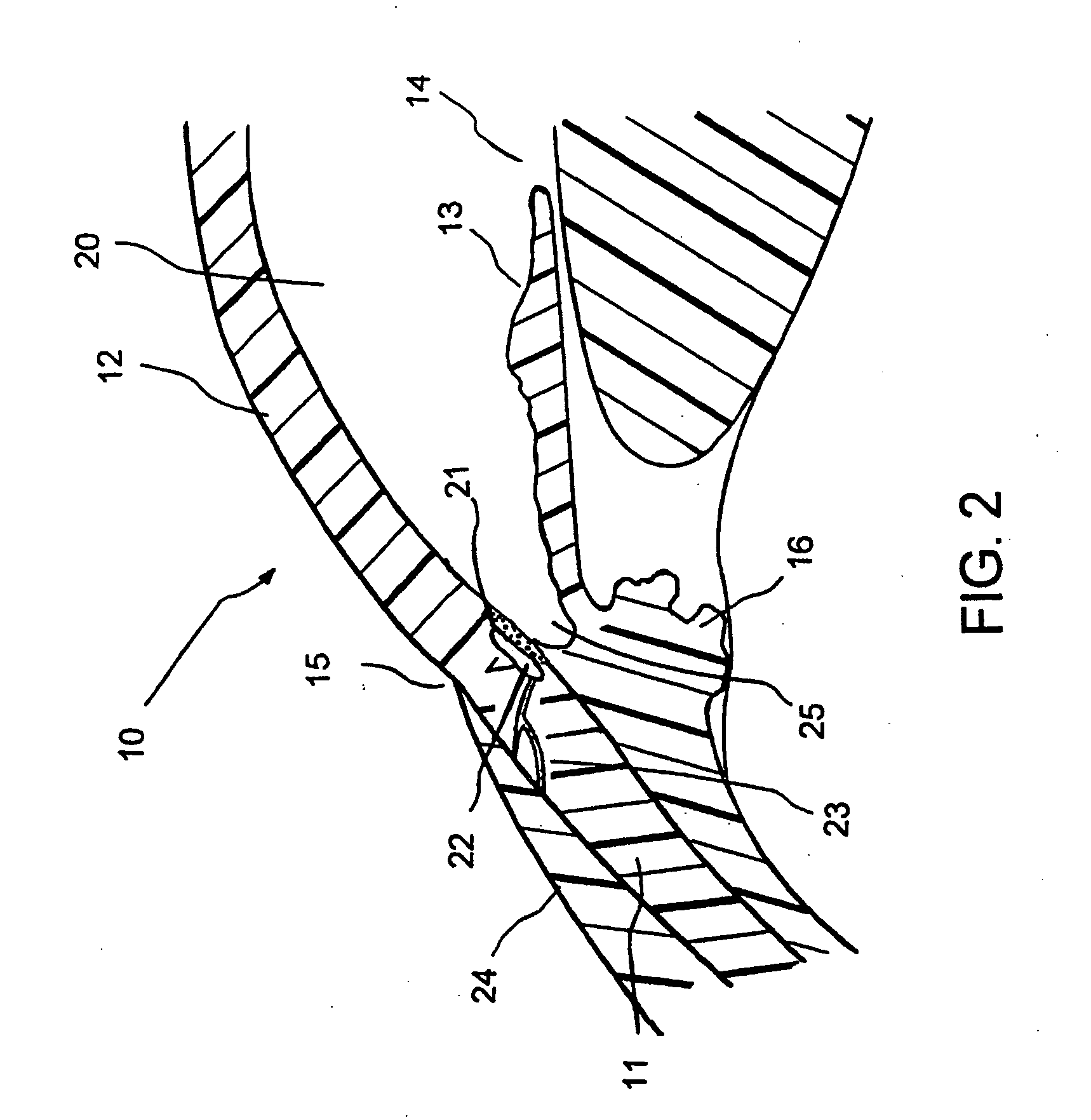
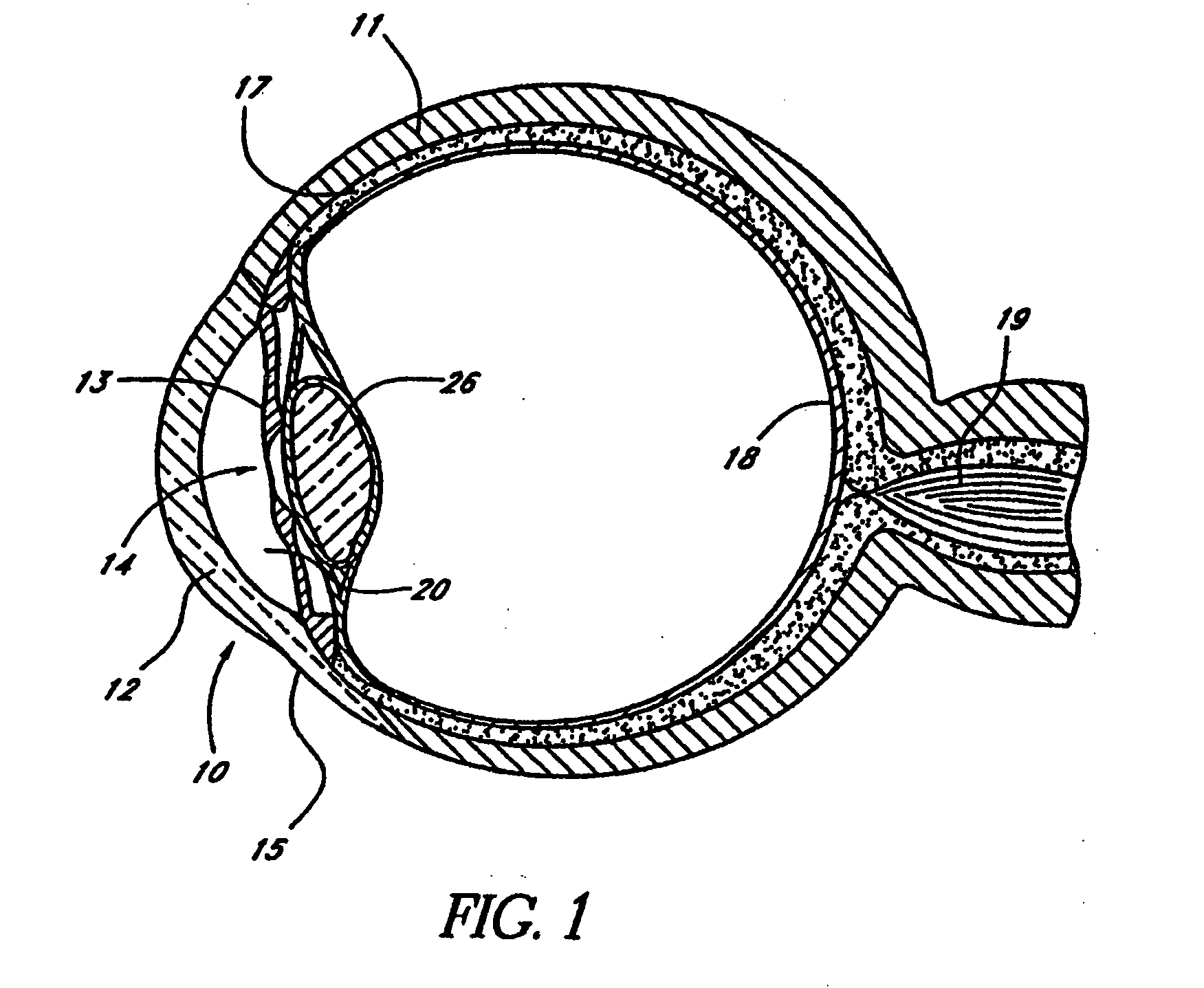
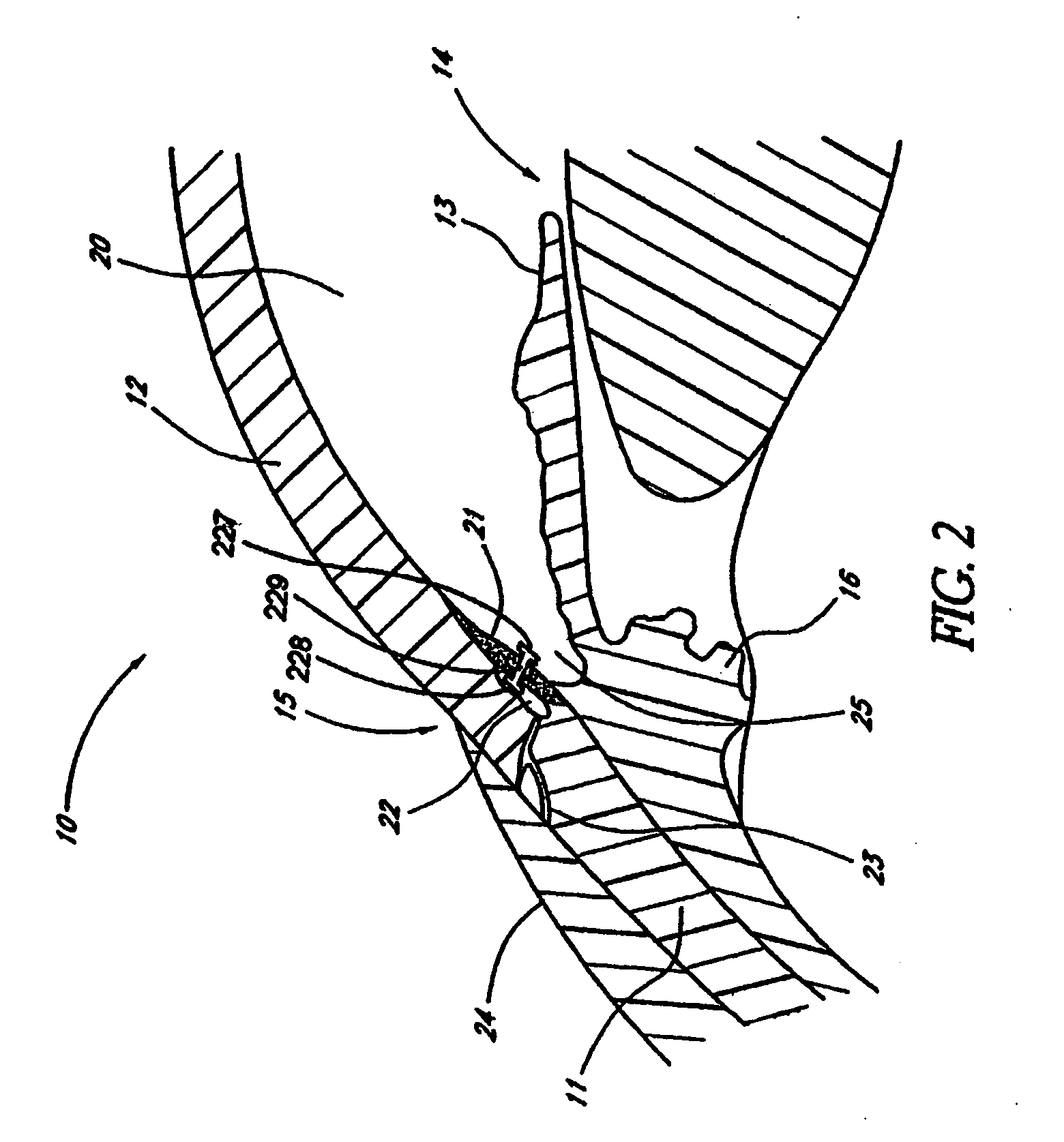
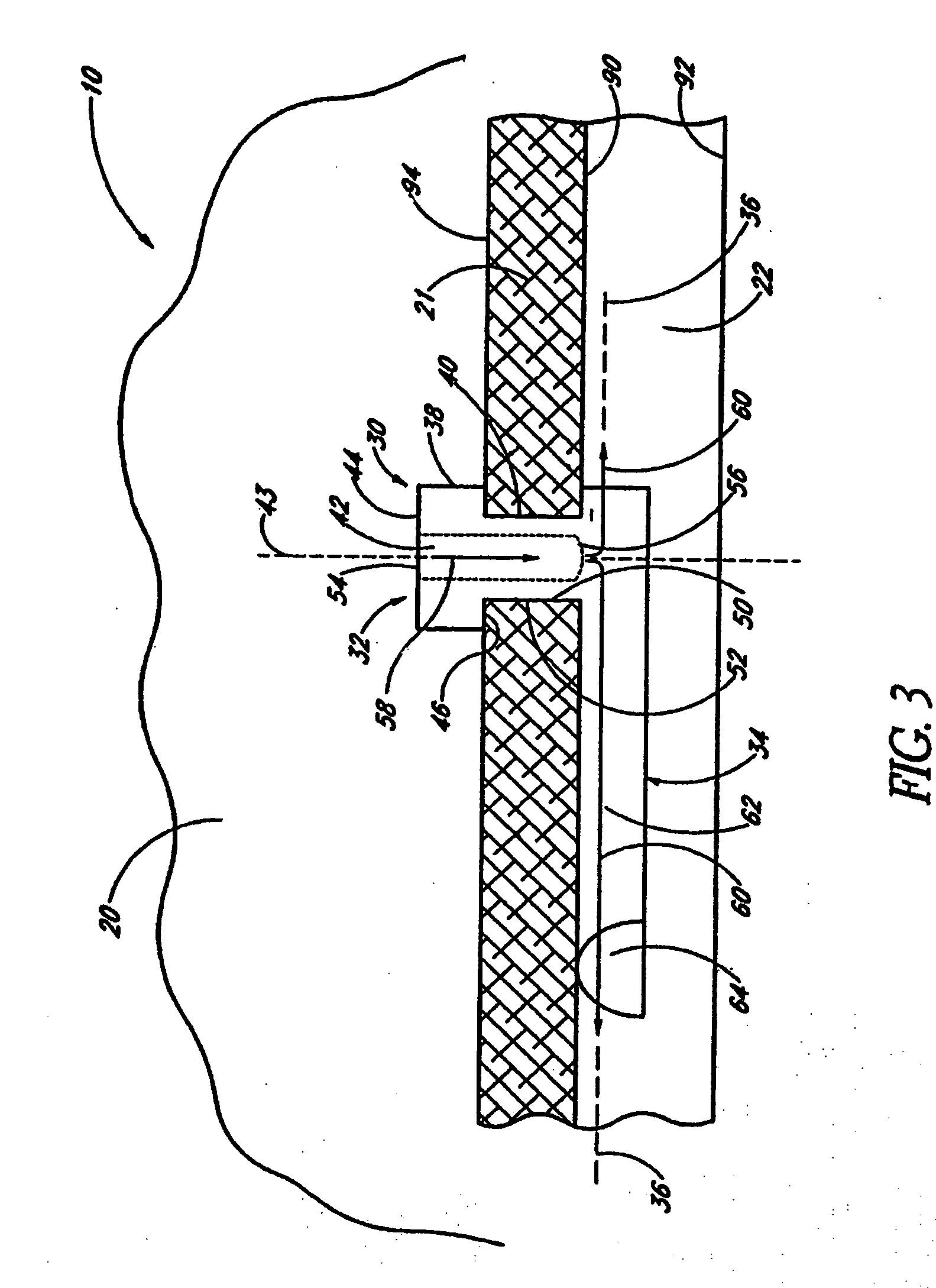
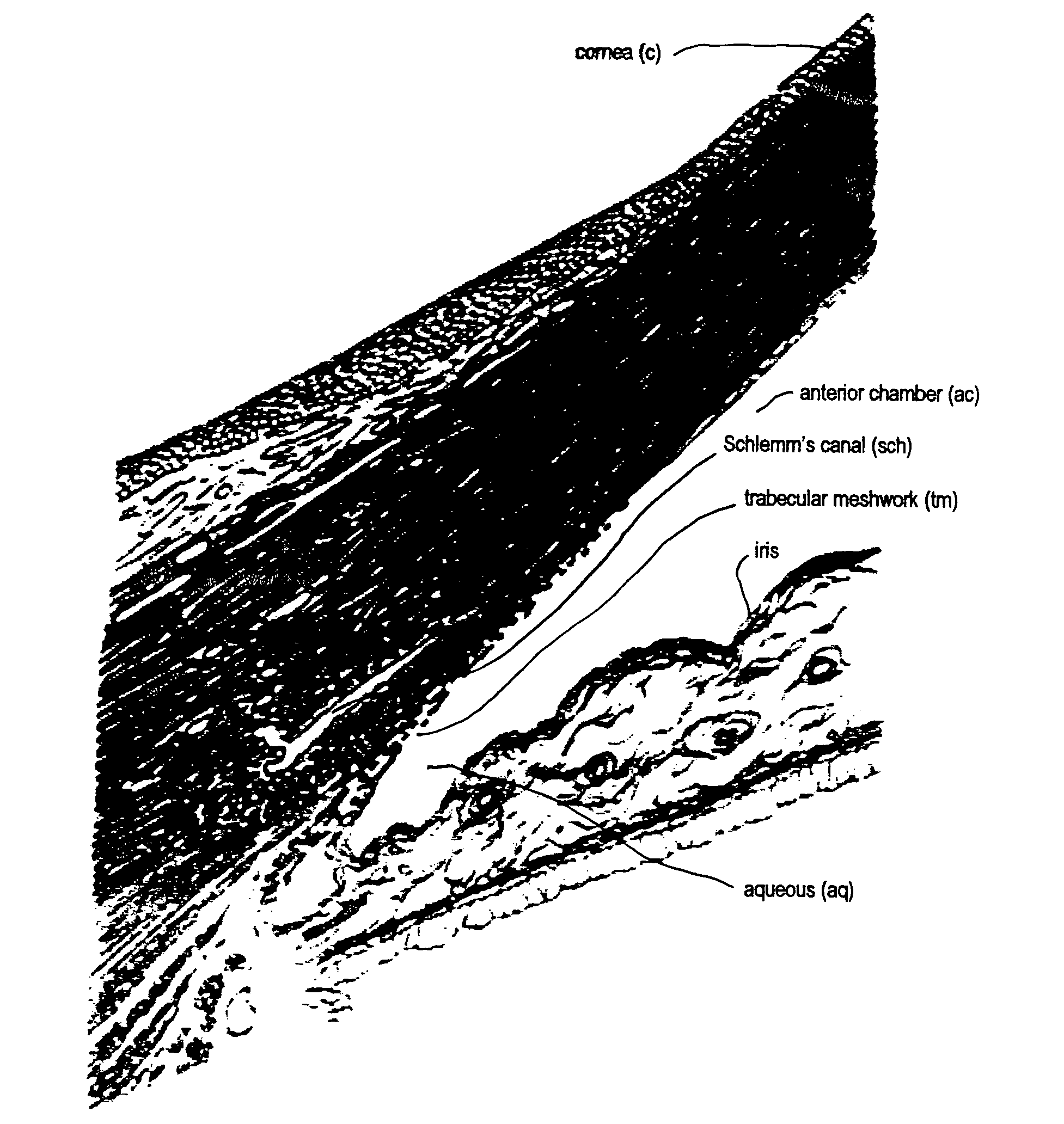
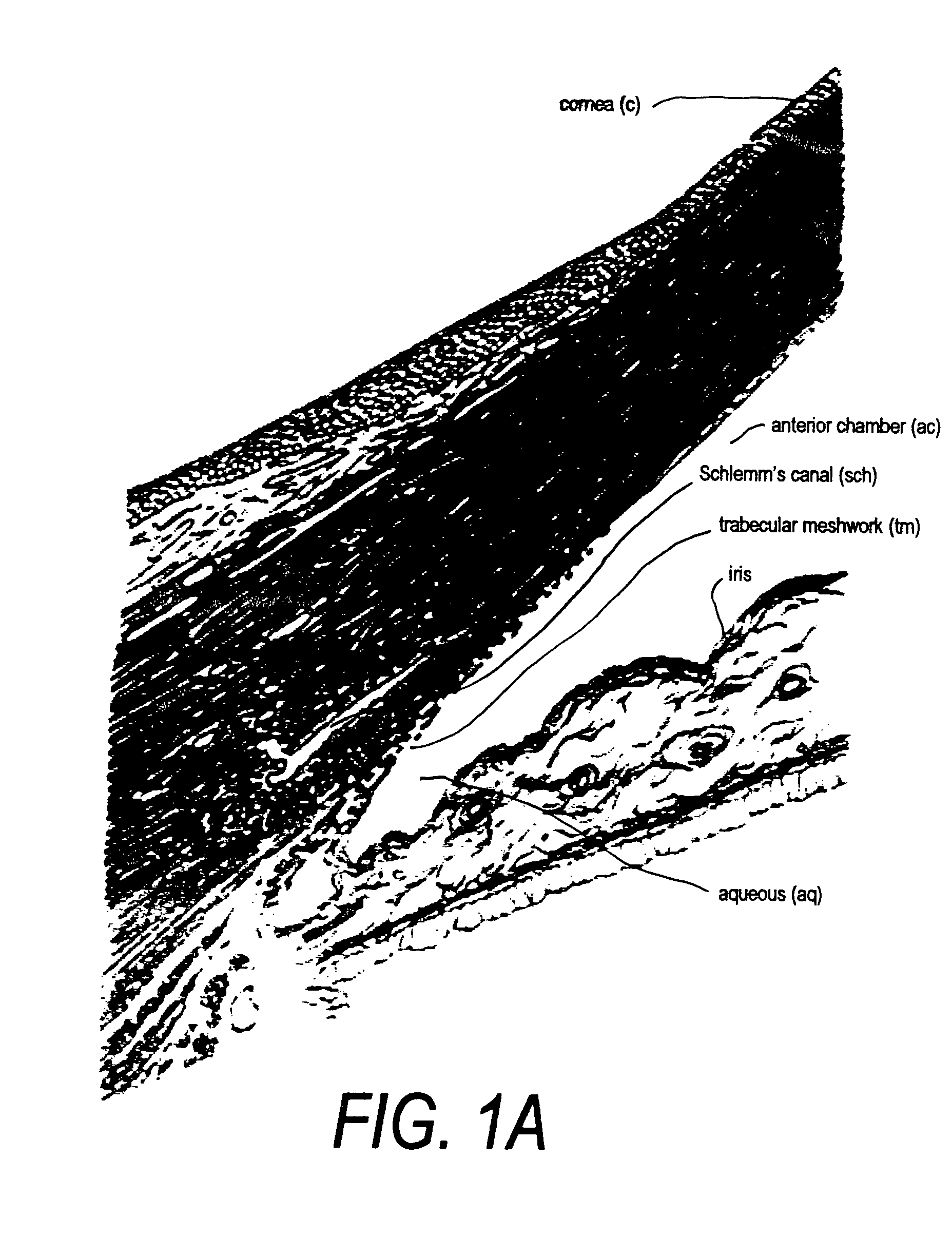
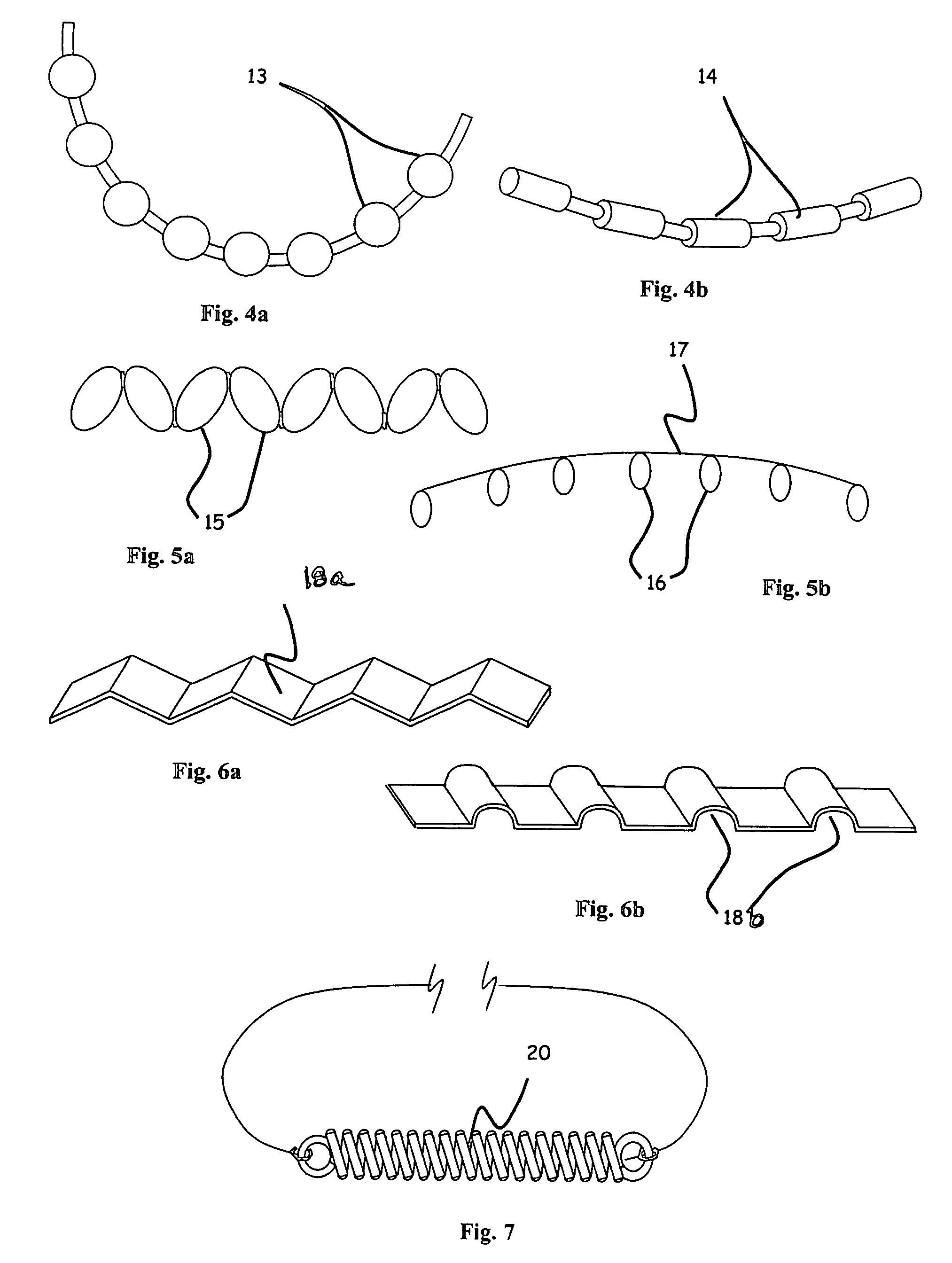
Disclosed is an implant for use in an eye with glaucoma, the implant including an inlet section in fluid communication with an outlet section, the inlet section being sized and shaped to fit at least partially in the anterior chamber of the eye, and the outlet section being sized and shaped to fit at least partially in Schlemm's canal of the eye. The implant also includes an expandable substrate suitable for expansion in the eye to assist in retaining the implant in the eye.
Owner:GLAUKOS CORP
Glaucoma treatment kit
A glaucoma treatment kit, containing intraocular stents and applicators, is disclosed. The stents are configured to extend between the anterior chamber and Schlemm's canal of the eye, for enhancing outflow of aqueous from the anterior chamber so as to reduce intraocular pressure.
Owner:GLAUKOS CORP
Ophthalmology implants and methods of manufacture
The present disclosure provides an ophthalmology implant and methods for treating glaucoma or optic neural transmission deficiency, wherein at least a portion of the implant is made of or includes a nanometer-sized substance, such as nanotubes, nanofibers, sheets from nanotubes, nanowires, nanofibrous mesh and the like.
Owner:GLAUKOS CORP
Injectable glaucoma implants with multiple openings
InactiveUS20050266047A1Faster and safe and less-expensive surgical procedureRapid visual recoveryOrganic active ingredientsEye surgerySchlemm's canalImplant
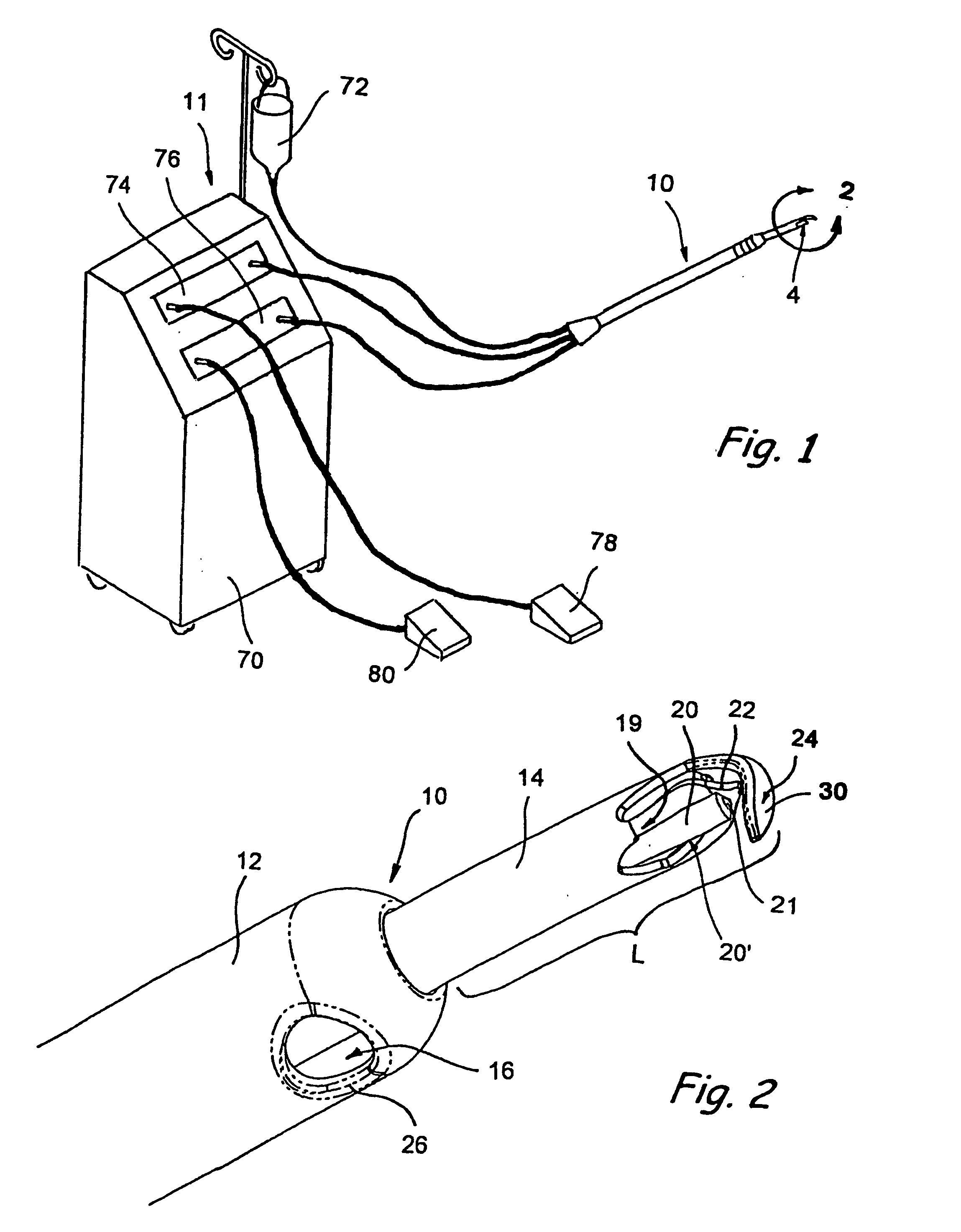
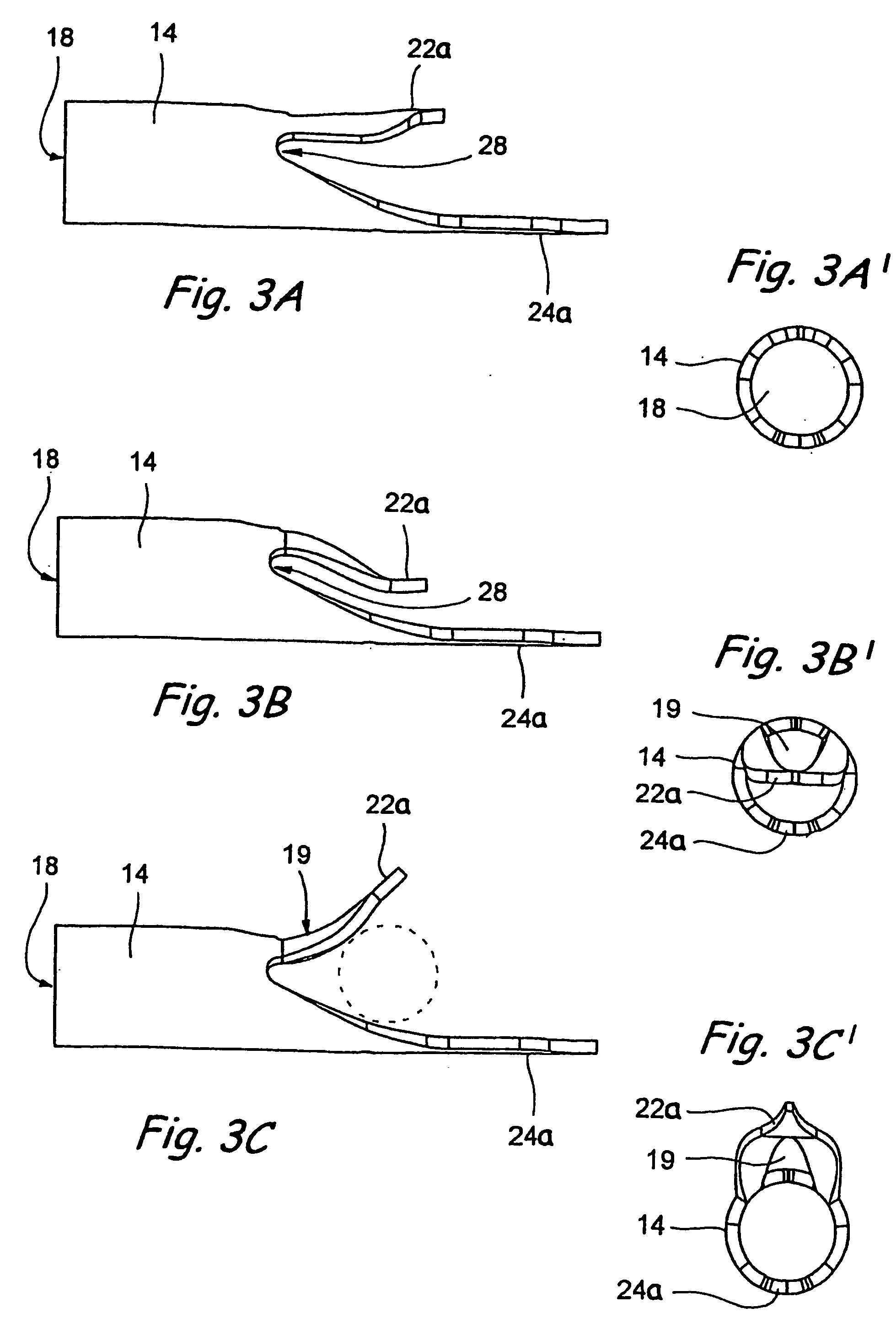
Intraocular stents and applicators are disclosed for treating glaucoma. The stents are configured to extend between the anterior chamber of the eye and Schlemm's canal for enhancing outflow of aqueous from the anterior chamber so as to reduce intraocular pressure. The stents can have features for anchoring the stent into Schlemm's canal as well as preventing the walls of Schlemm's canal from closing the outlet of the stents. The applicators can be steerable so as to make implantation easier. Additionally, the applicators can be configured to hold a plurality of stents so that multiple stents can be implanted through one incision without removing the applicator from the incision between serial implantations.
Owner:GLAUKOS CORP
Devices and techniques for treating glaucoma
InactiveUS6989007B2Improve facilitiesGood mannersLaser surgeryDiagnosticsOpen angle glaucomaAqueous outflow
A system for non-invasive treatment of a patient's trabecular meshwork to treat primary open-angle glaucoma. The system and technique applies energy directly to media within clogged spaces in a patient's trabecular meshwork to increase aqueous outflow facility by (i) localization of microimplantable bodies carrying a selected exogenous chromophore, such as particles with a gold surface, in deeper regions of the trabecular meshwork, and (ii) irradiation of the microimplantables with a selected coherent wavelength having a power level and pulse duration that is strongly absorbed by the surfaces of the microimplantables.
Owner:OCCULOGIX CORP
Device and methods useable for treatment of glaucoma and other surgical procedures
InactiveUS20060241580A1Not to damageAvoiding significant and irreparable damageEye surgerySurgical instruments for heatingSurgical departmentTissue protection
A device and method for cutting or ablating tissue in a human or veterinary patient includes an elongate probe having a distal end, a tissue cutting or ablating apparatus located adjacent within the distal end, and a tissue protector extending from the distal end. The protector generally has a first side and a second side and the tissue cutting or ablating apparatus is located adjacent to the first side thereof. The distal end is structured to be advanceable into tissue or otherwise placed and positioned within the patient's body such that tissue adjacent to the first side of the protector is cut away or ablated by the tissue cutting or ablation apparatus while tissue that is adjacent to the second side of the protector is not substantially damaged by the tissue cutting or ablating apparatus.
Owner:SHOWA DENKO KK +1
Apparatus and method for surgical bypass of aqueous humor
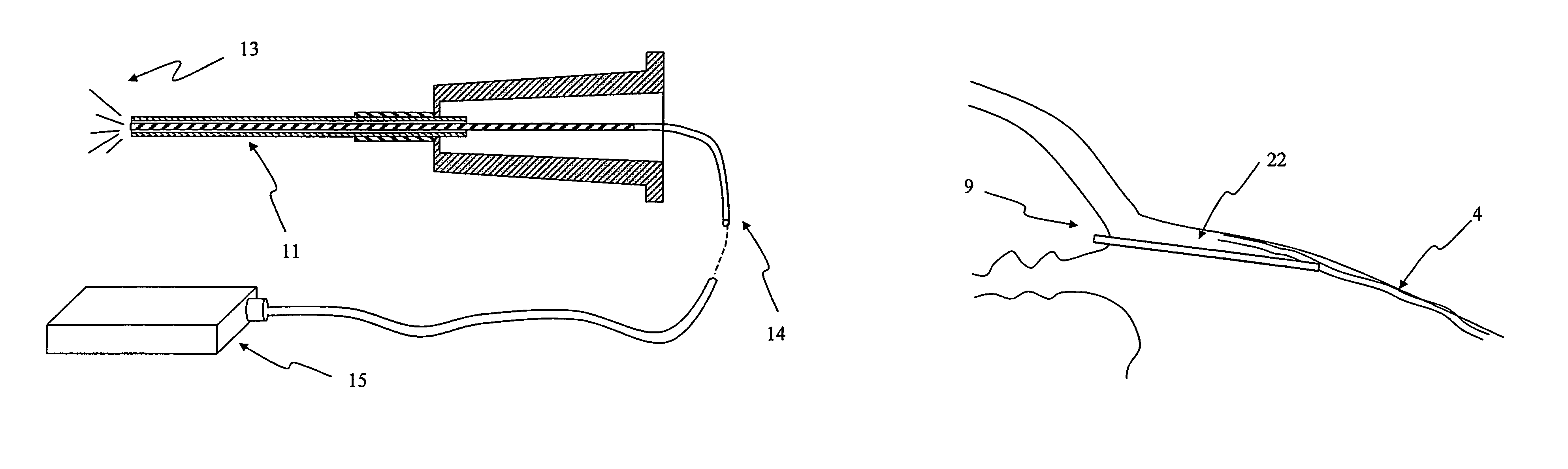
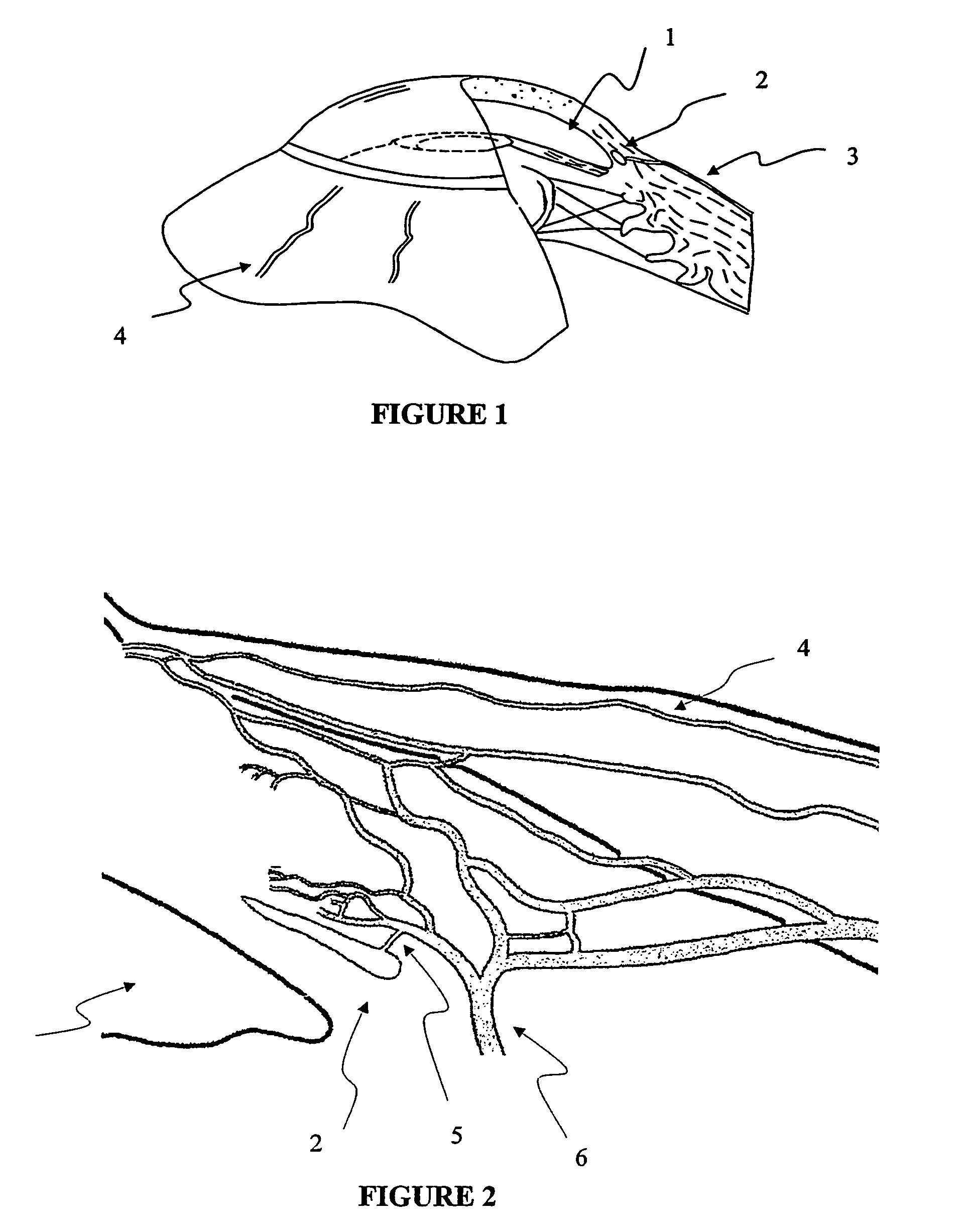
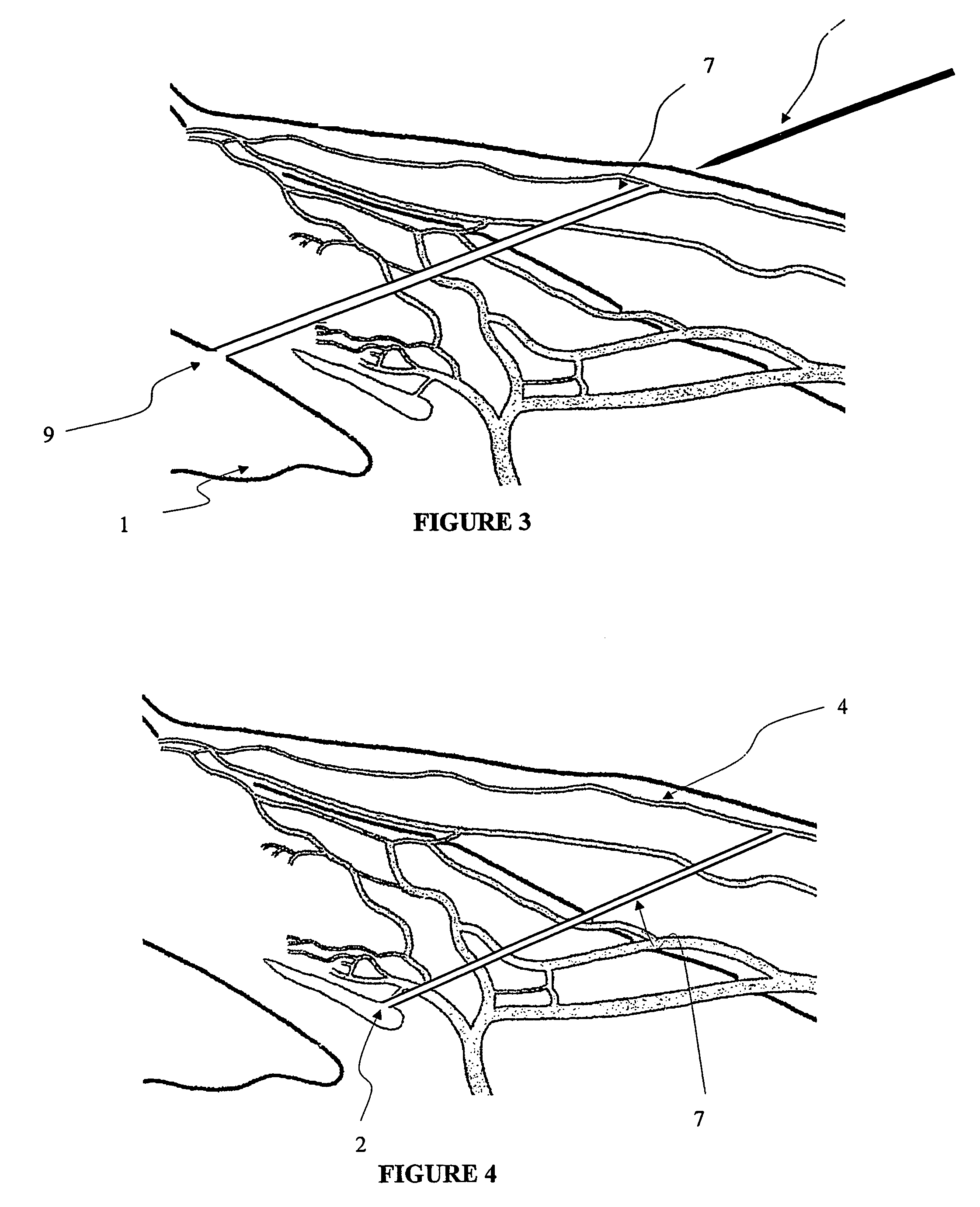
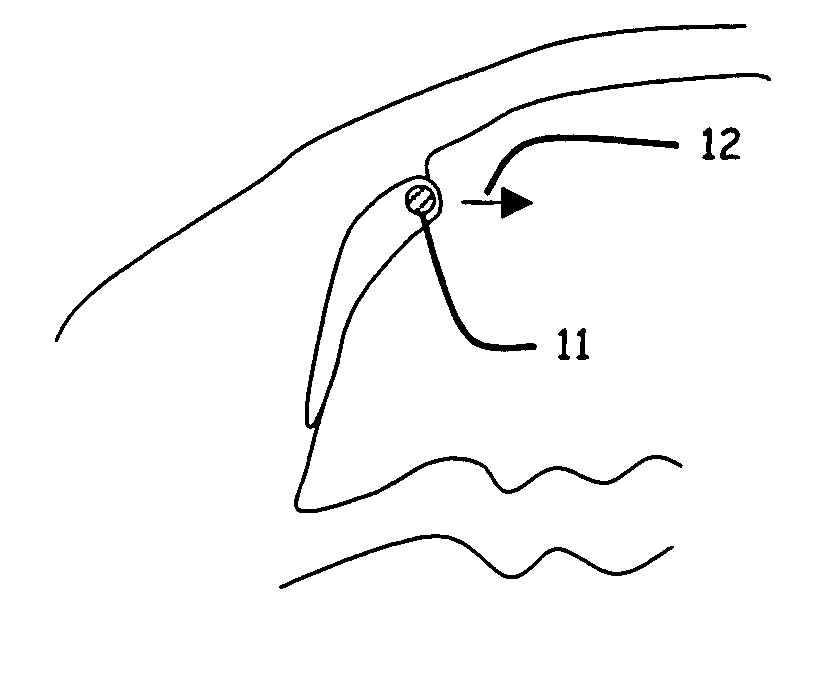
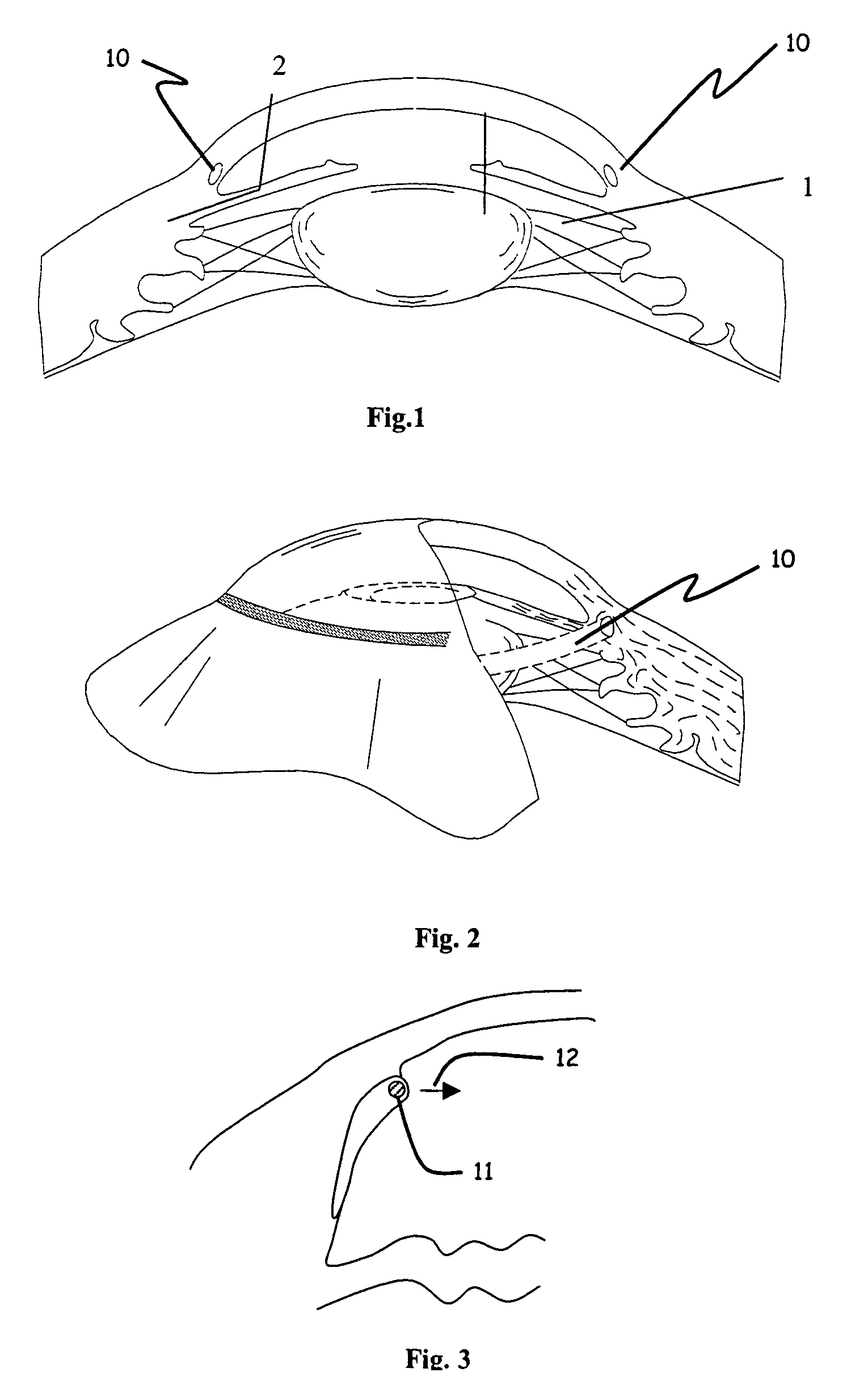
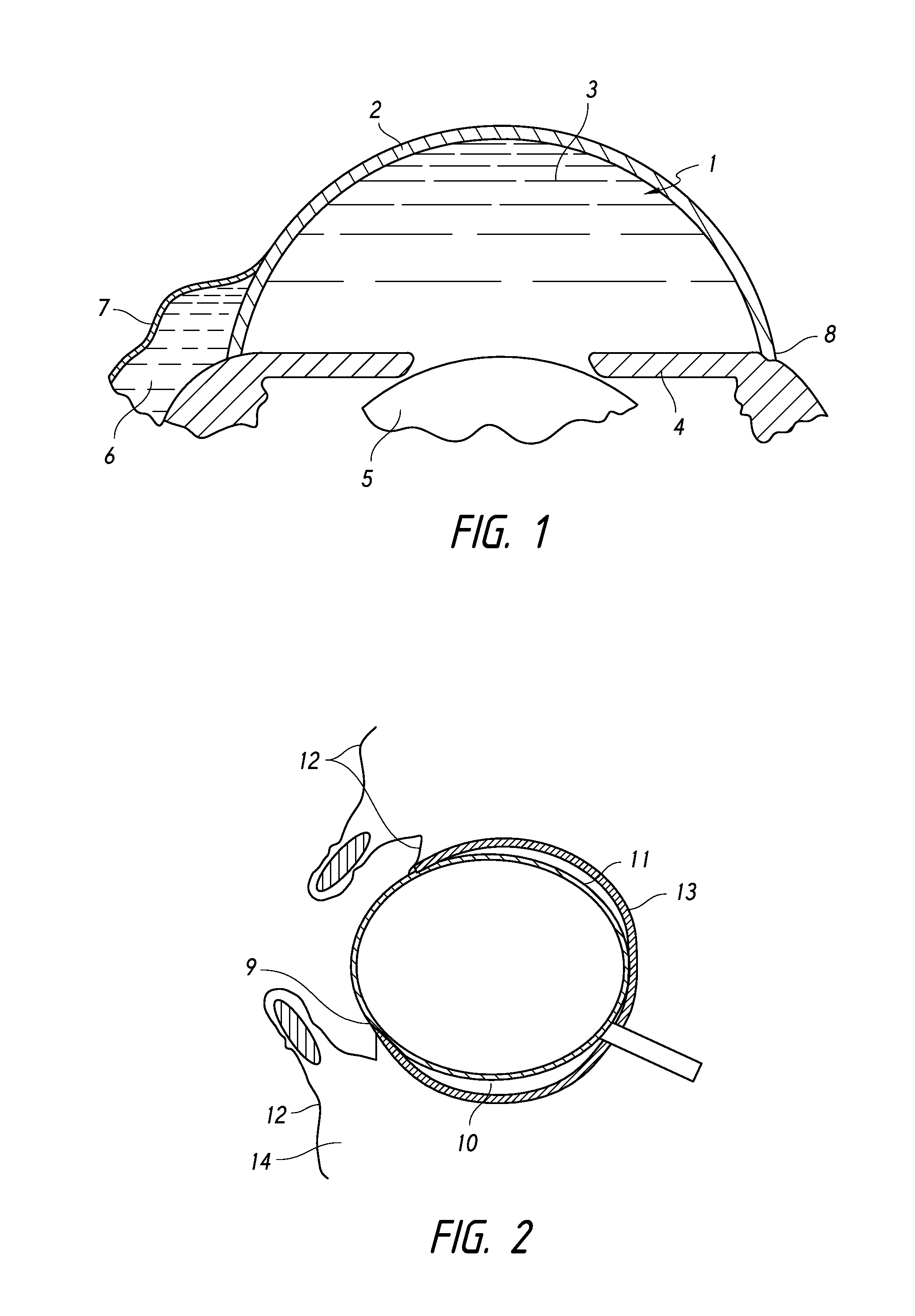
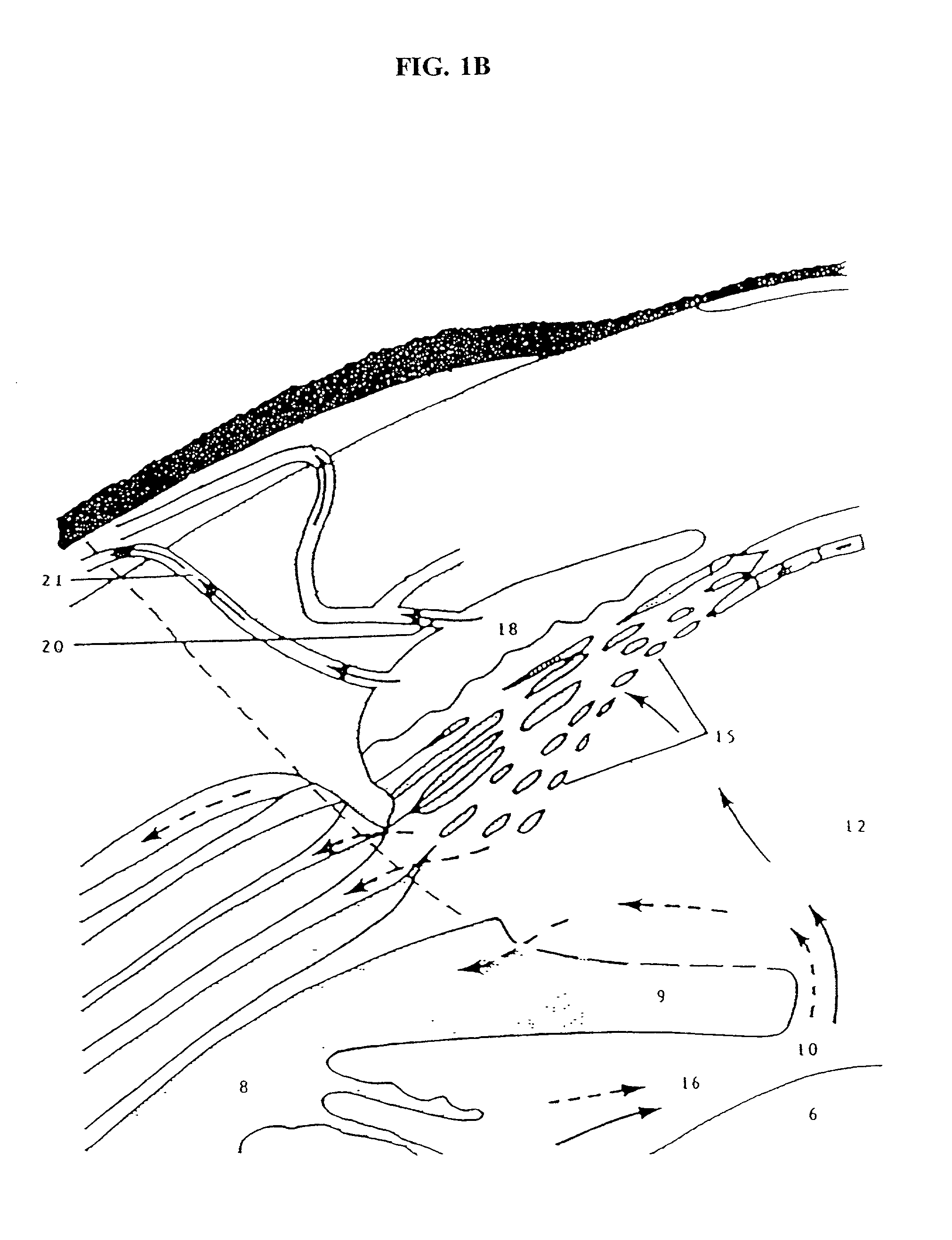
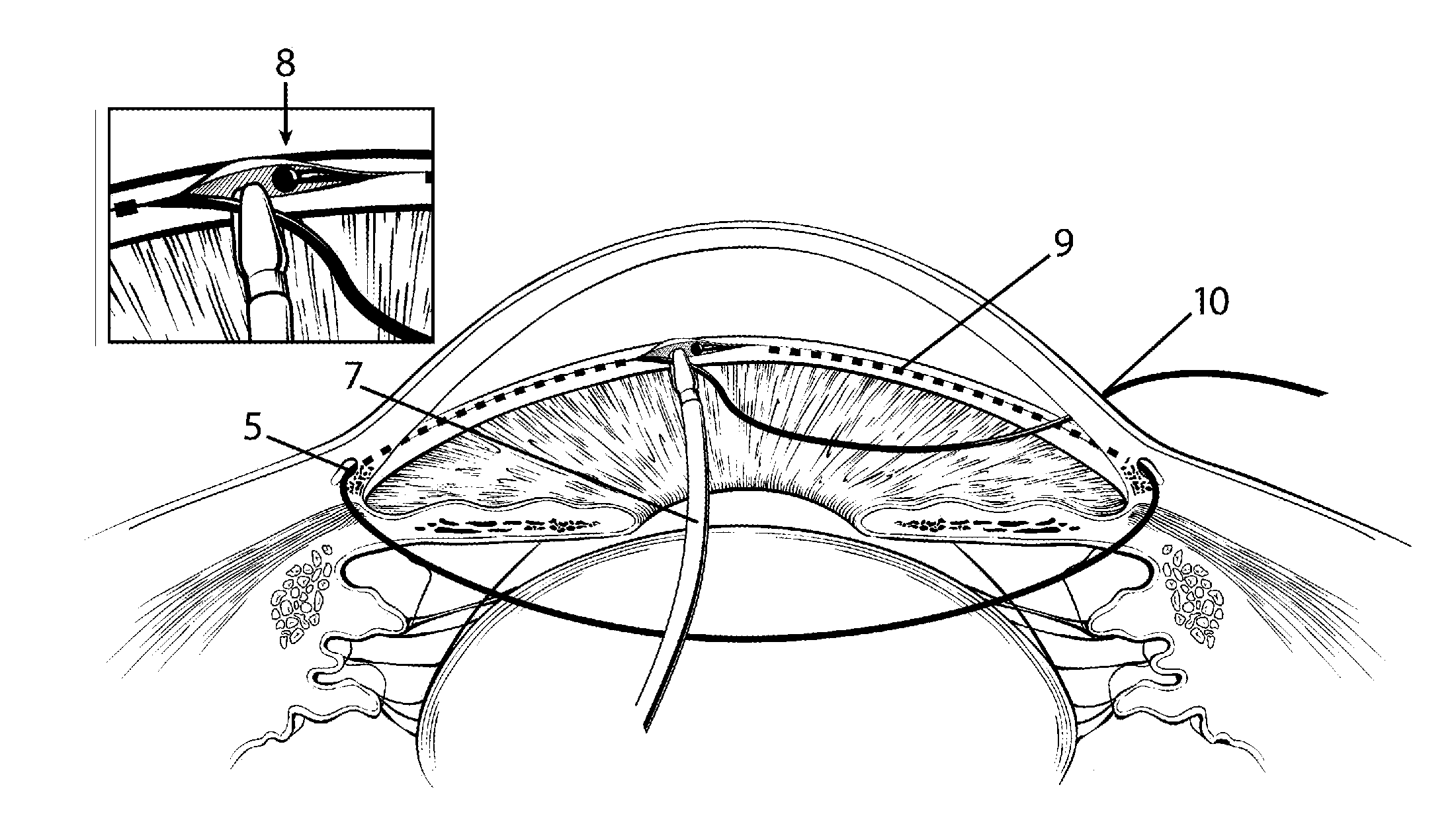
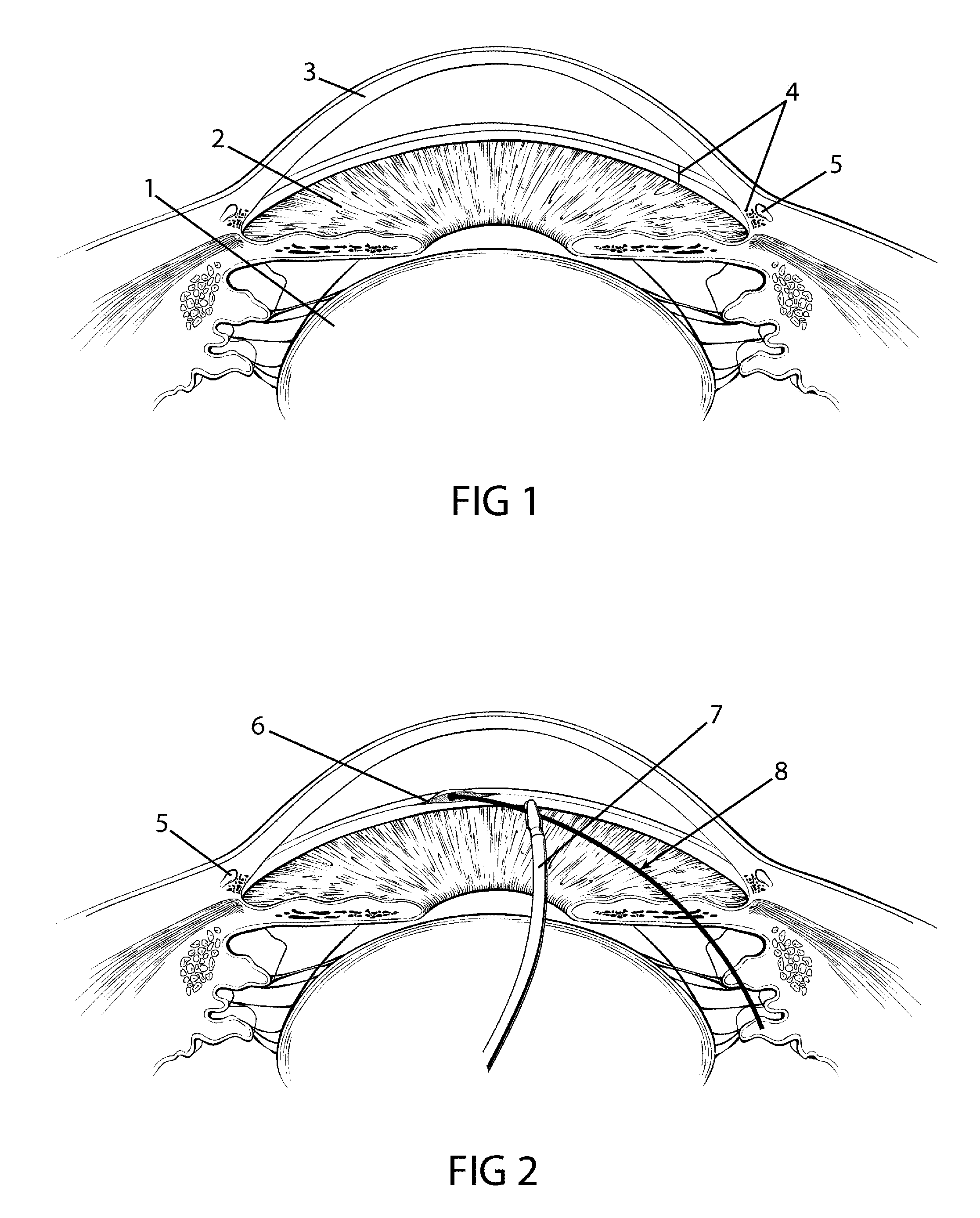
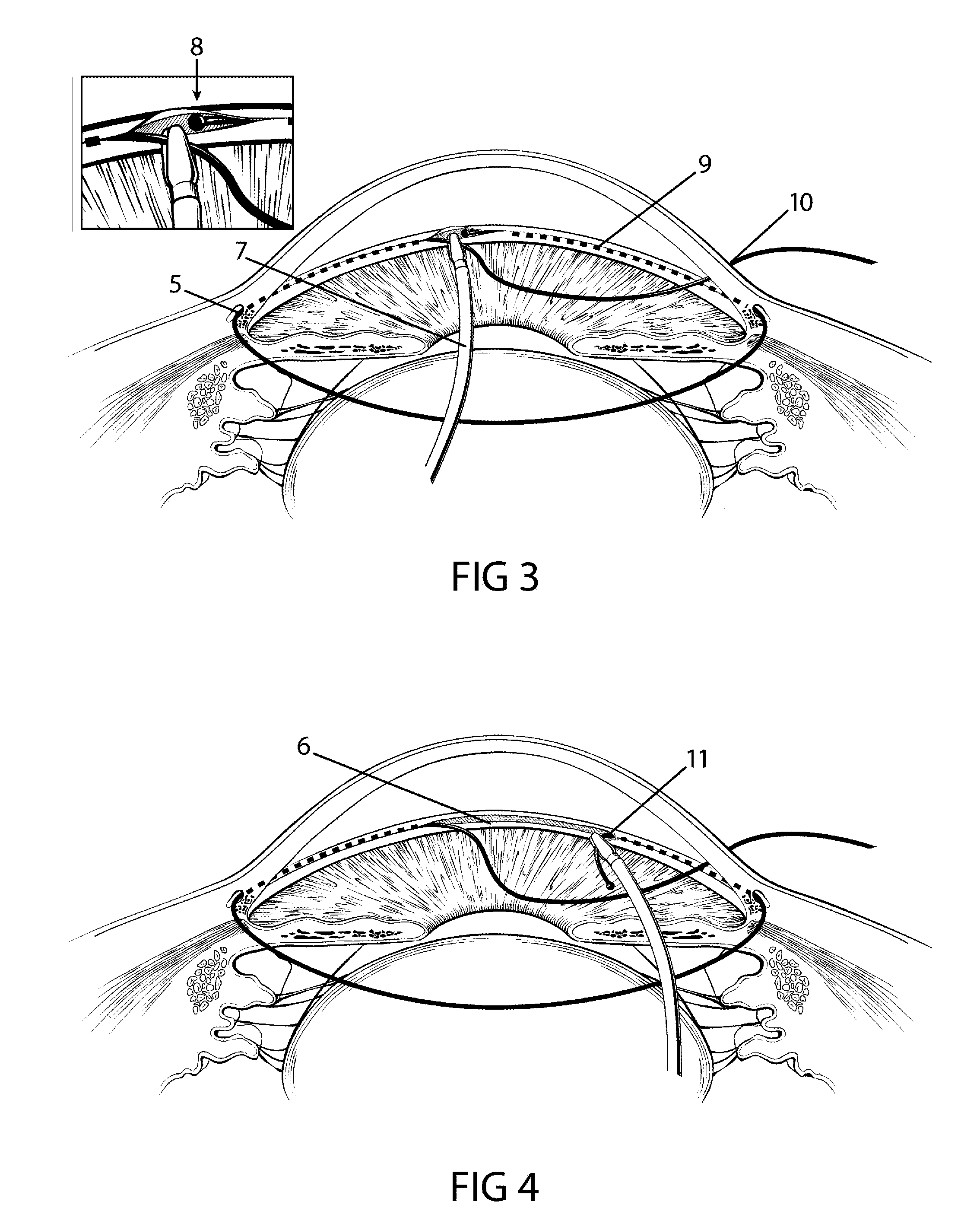
The invention provides minimally invasive microsurgical tools and methods to form an aqueous humor shunt or bypass for the treatment of glaucoma. The invention enables surgical creation of a tissue tract (7) within the tissues of the eye to directly connect a source of aqueous humor such as the anterior chamber (1), to an ocular vein (4). The tissue tract (7) from the vein (4) may be connected to any source of aqueous humor, including the anterior chamber (1), an aqueous collector channel, Schlemm's canal (2), or a drainage bleb. Since the aqueous humor passes directly into the venous system, the normal drainage process for aqueous humor is restored. Furthermore, the invention discloses devices and materials that can be implanted in the tissue tract to maintain the tissue space and fluid flow.
Owner:ISCI INTERVENTIONAL CORP
Glaucoma Treatment Device
InactiveUS20110306915A1Minimize scarringRemove complicationsEye implantsEar treatmentFlow diverterSuprachoroidal space
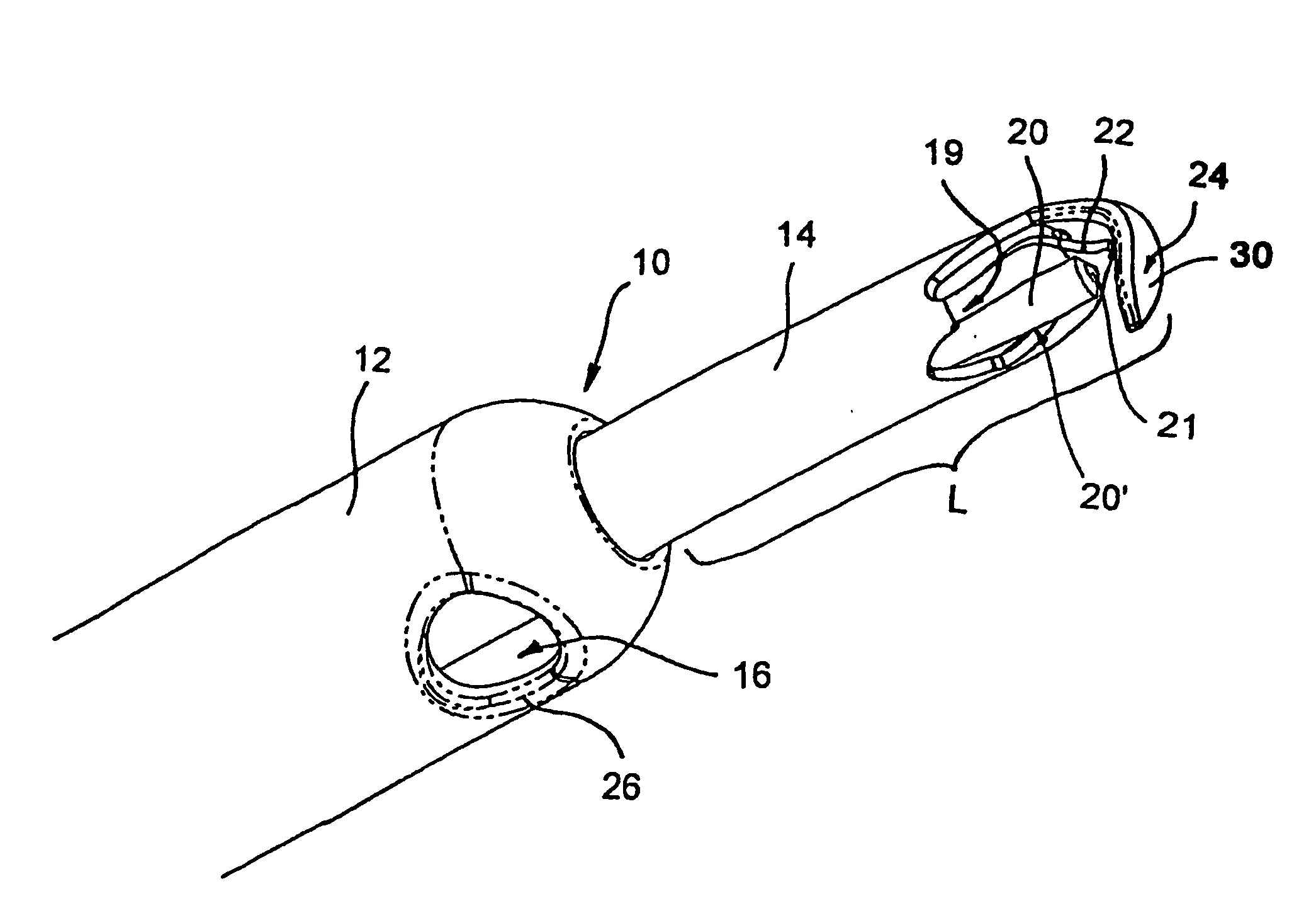
Methods and devices are adapted for implanting into the eye. An incision is formed in the cornea of the eye and a shunt is inserted through the incision into the anterior chamber of the eye. The shunt includes a fluid passageway. The shunt is passed along a pathway from the anterior chamber through the scleral spur of the eye into the suprachoroidal space and positioned in a first position such that a first portion of the fluid passageway communicates with the anterior chamber and a second portion of the fluid passageway communicates with the suprachoroidal space to provide a fluid passageway between the suprachoroidal space and the anterior chamber.
Owner:NOVARTIS AG
Ophthalmic implant for treatment of glaucoma
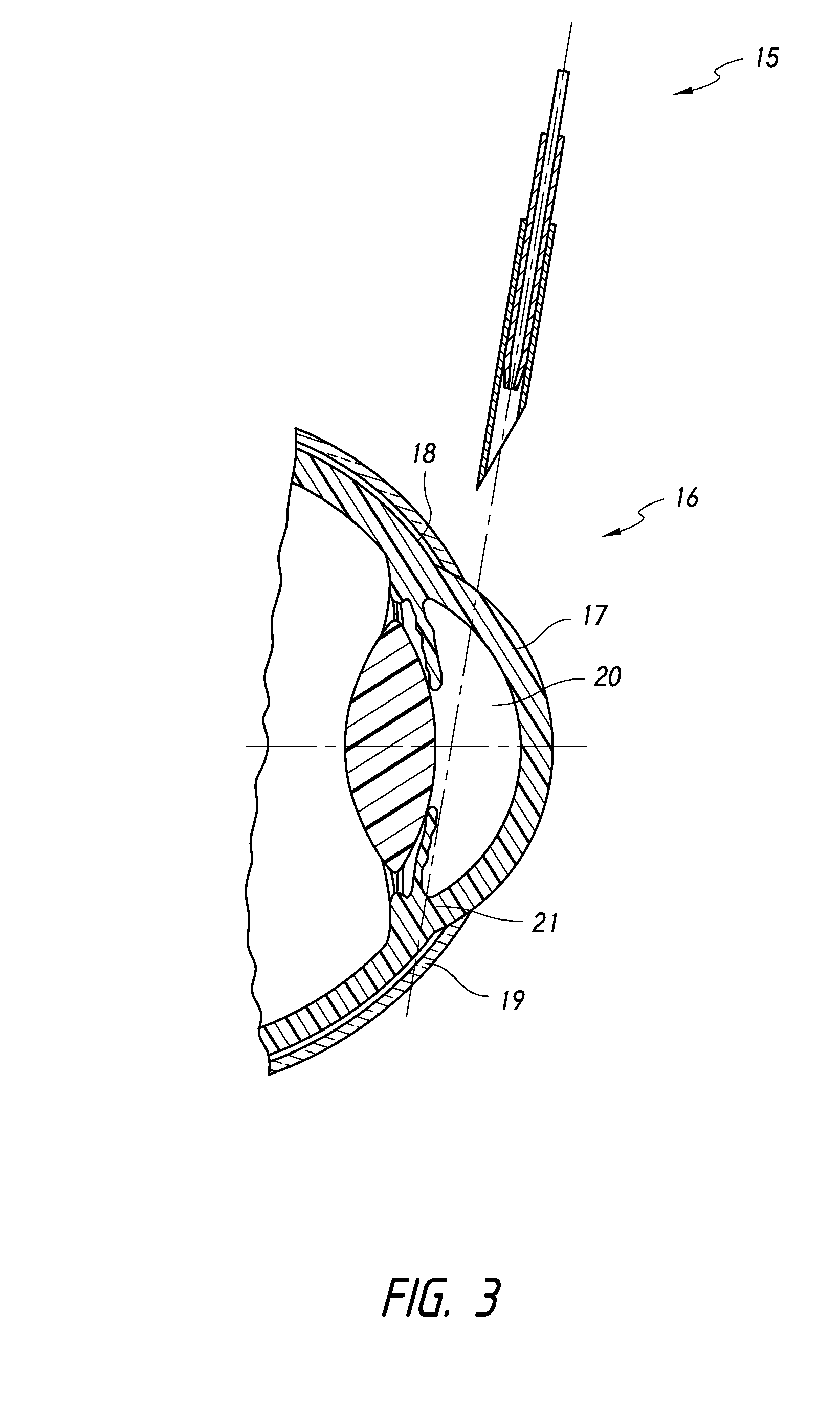
ActiveUS8034105B2Sufficient forceImprove permeabilityEye implantsEye surgeryOphthalmological implantSchlemm's canal
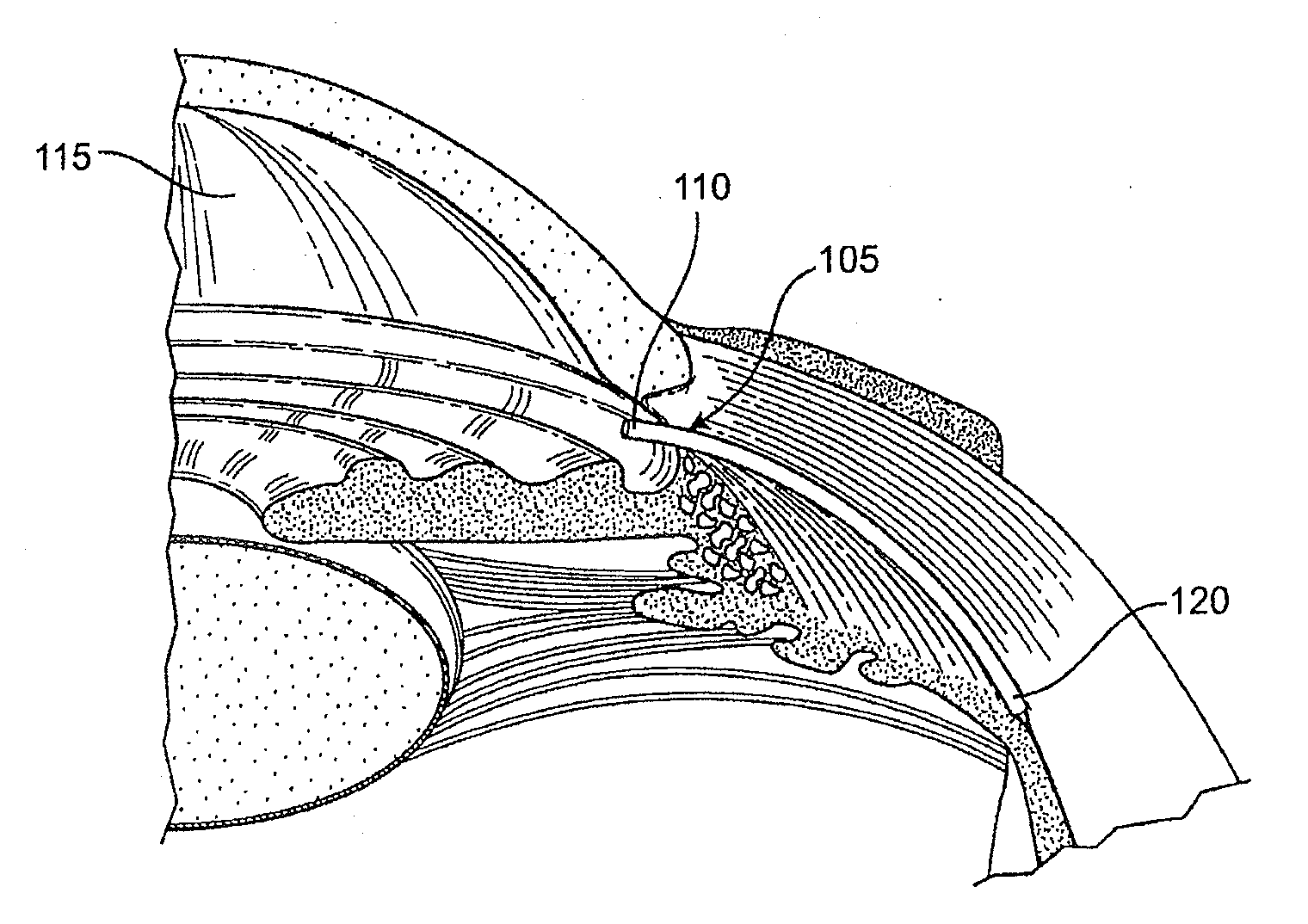
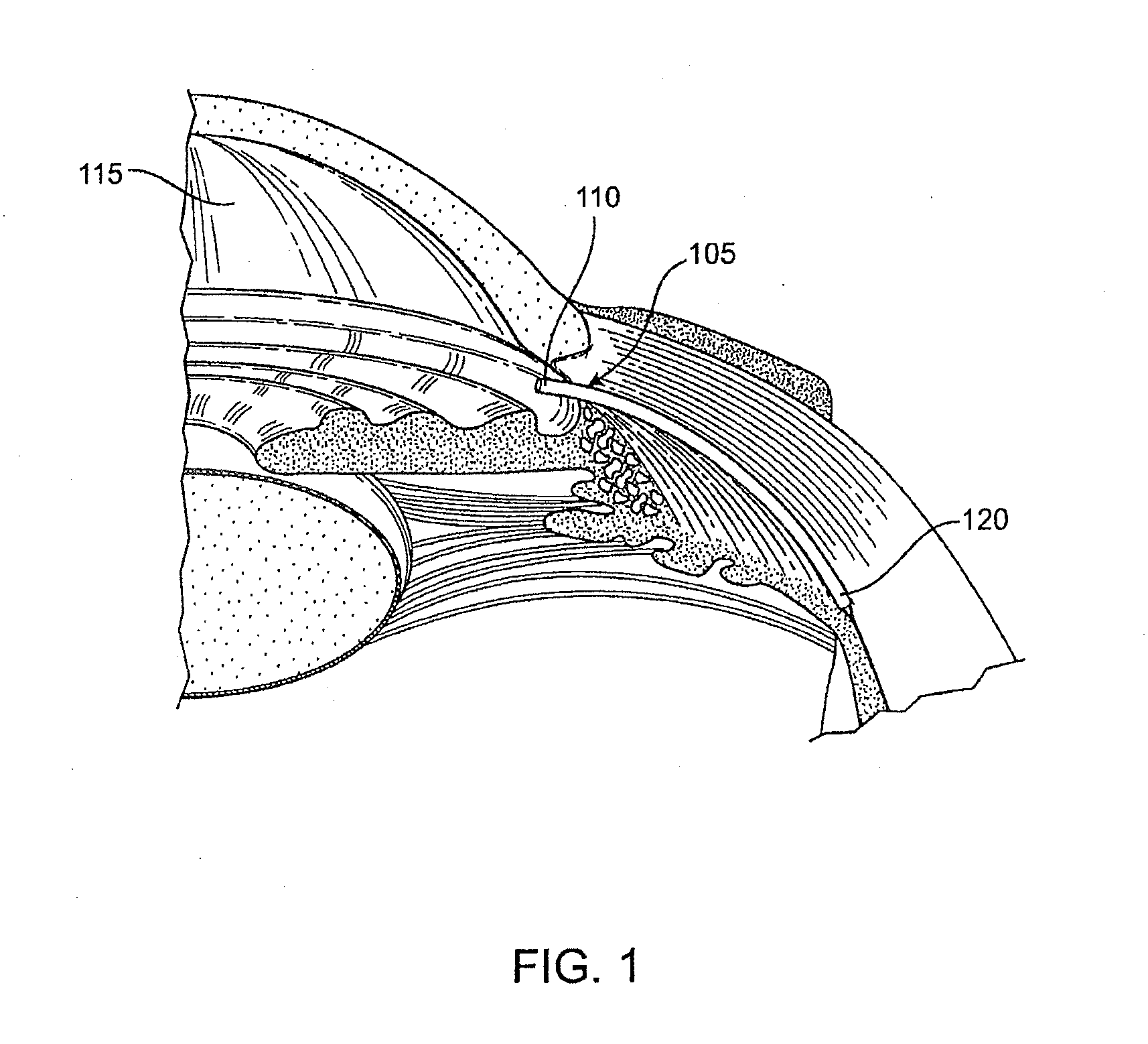
A method is provided for increasing the outflow of fluid through Schlemm's canal that is useful for treatment of glaucoma. The implant is placed in Schlemm's canal by use of a flexible delivery instrument attached to the implant. The instrument and implant are positioned within the canal, the implant is released and the distal and proximal ends of the implant are connected to apply sufficient axial tensioning force on the inner wall of the canal to increase fluid permeability. In another embodiment a delivery instrument attached to the implant is positioned in the canal securing one of the distal or proximal ends of the implant within the canal. The implant provides sufficient axial tensioning force on the inner wall of the canal to increase fluid permeability of the inner wall of the canal. The other of the distal or proximal ends may be secured to maintain the tensioning force on the inner wall of the canal.
Owner:NOVA EYE INC
Composition and method for reducing the risk or progression of cardiovascular, glaucoma, tardive dyskinesia and other diseases
InactiveUS20040087479A1Reduce riskShorten the progressBiocideOrganic active ingredientsBeta-CaroteneAdditive ingredient
Elevated levels of homocysteine have been implicated as an important risk factor for cardiovascular and other diseases. A composition for decreasing levels of plasma homocysteine and a method for administering the composition are provided, the composition containing dextromethorphan (DM), folic acid and vitamins B6 and B12. The composition provides a synergistic therapeutic effect so that lower amounts of the above ingredients may be employed to minimize any undesirable side effects caused by the use of high levels of a component such as DM. Preferred compositions for cardiovascular diseases further include lecithin, vitamin E, betacarotene, procyanidins / flavonoids, trimethylglycine, garlic oil and minerals. Other compositions for treating glaucoma include bilberry, bioflavonoids and beta-carotene and for treating tardive dyskinesia include an antioxidant such a grape seed extract and pine bark extract, lecithin and oligomeric proanthocyanidins. The compositions may be administered using any suitable means such as orally or intravenous.
Owner:SOSNOWSKI ROBERT E +1
Intraocular devices
ActiveUS20150011926A1Safeguarding integrityReduces eliminates riskEar treatmentEye surgeryTreatment glaucomaOphthalmology
Glaucoma can be treated by implanting an intraocular shunt into the eye. Such procedures can employ various deployment devices, shunts, and implantation techniques.
Owner:AQUESYS INC
Compositions and methods for treating diseases
This invention relates to compositions and methods for treatment of vascular conditions. The invention provides arginine polymers and arginine homopolymers for the treatment and / or prevention of glaucoma, pulmonary hypertension, asthma, chronic obstructive pulmonary disease, erectile dysfunction, Raynaud's syndrome, heparin overdose, vulvodynia, and wound healing. The invention also provides arginine polymers and arginine homopolymers for use in organ perfusate and preservation solutions.
Owner:LUMEN THERAPEUTICS
Methods and compositions for treating diabetes, metabolic syndrome and other conditions
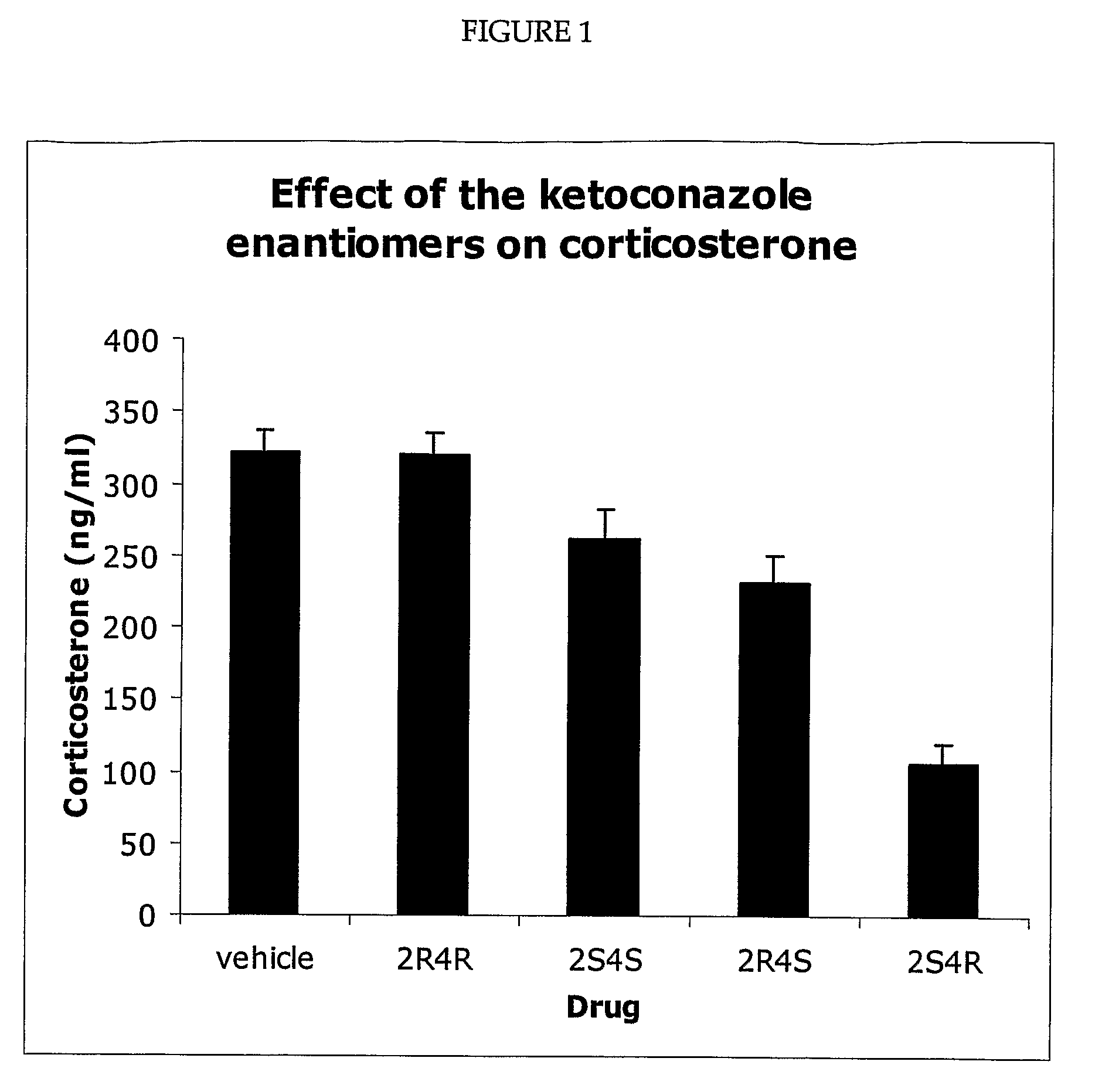
InactiveUS20090233843A1Reduce concentrationBiocideOrganic active ingredientsInsulin resistanceType 2 diabetes
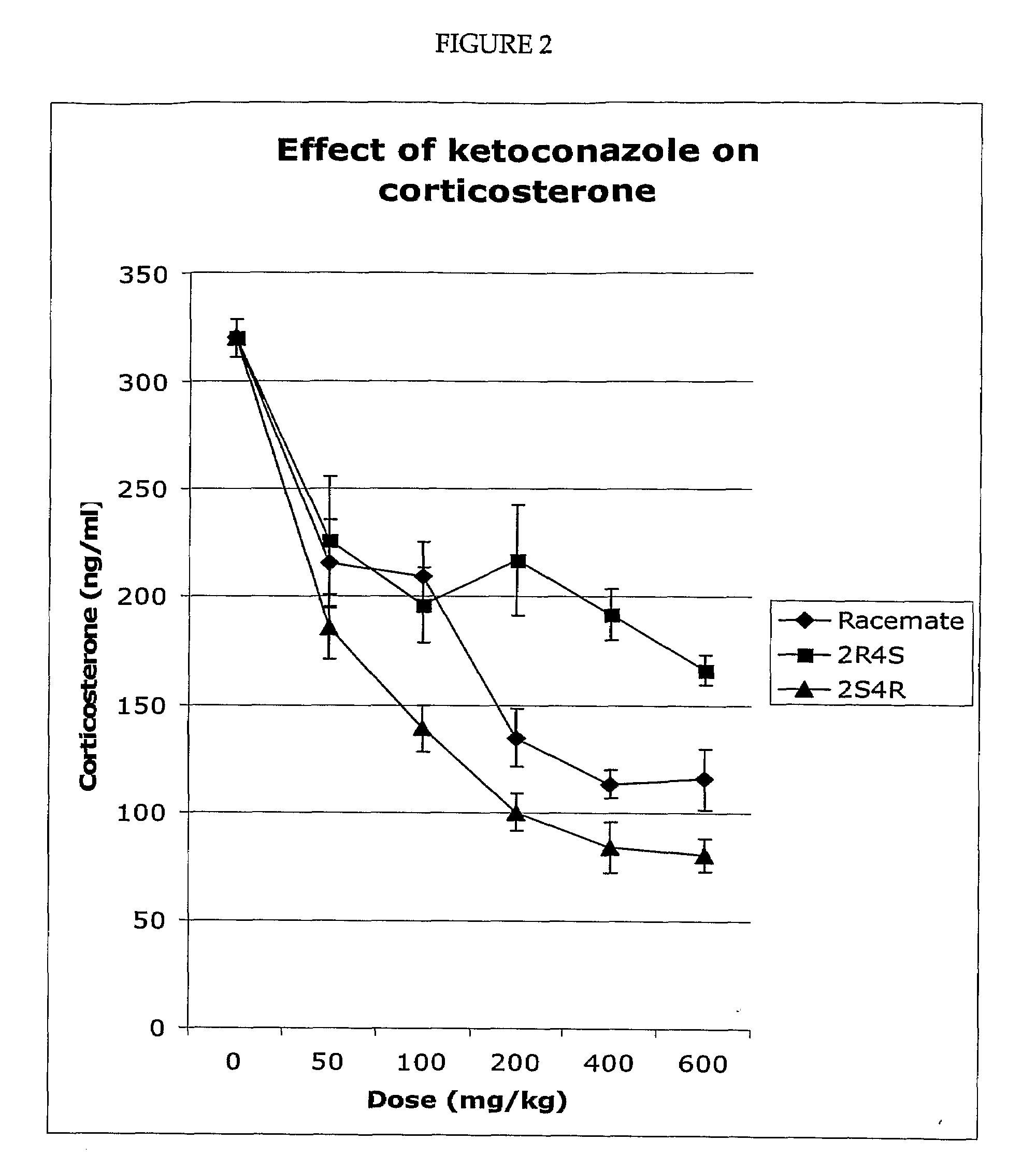
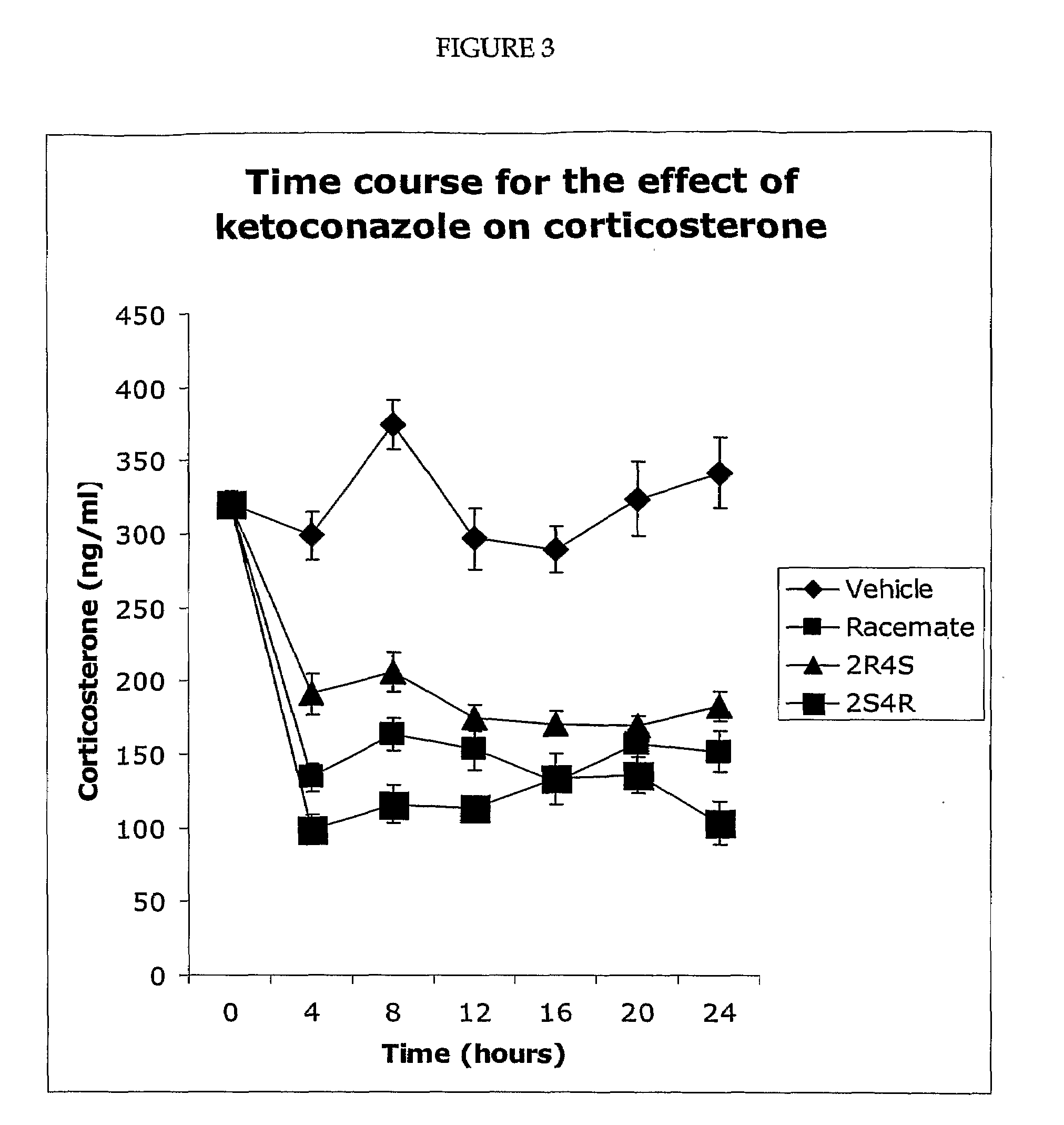
Pharmaceuticals compositions comprising the 2S, 4R, ketoconazole enantiomer or its pharmaceutically acceptable salts, hydrates, and solvates, that are substantially free of the 2R, 4S ketoconazole enantiomer are useful to reduce cortisol synthese and for the treatment of type 2 diabetes, hyperglycemia, obesity, insulin resistance, dyslipidemia, hyperlipidemia, hypertension, Metabolic Syndrome, and other diseases and conditions, including but not limited to Cushing's Syndrome, depression, and glaucoma.
Owner:CORTENDO
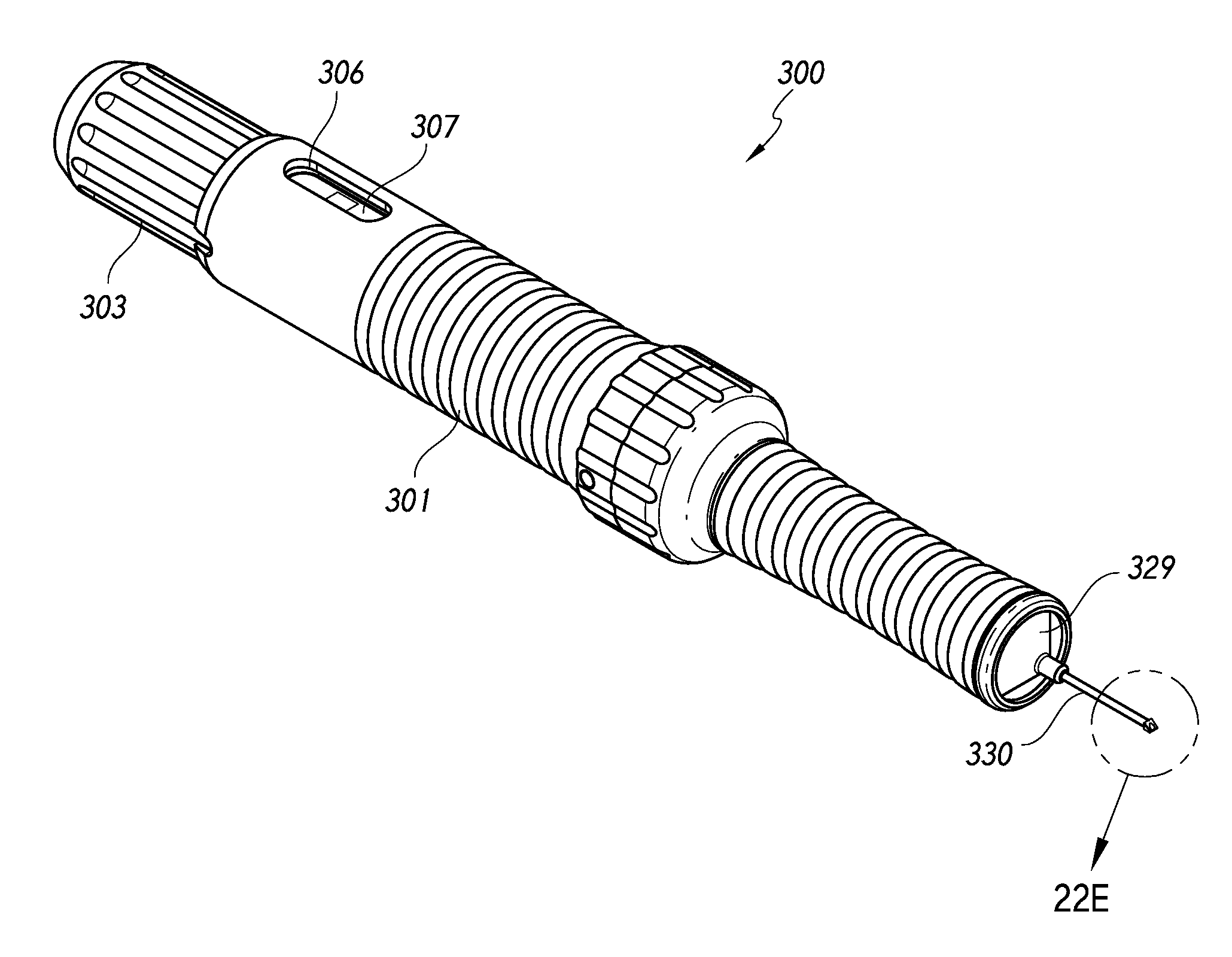
Intraocular shunt inserter
An inserter for treating glaucoma can comprise a housing, a needle, a plunger, a slider component, and a drive component. The drive component is disposed within a cavity of the housing and rotatable within the cavity to result in movement along a longitudinal axis of the inserter to the needle and the plunger upon rotation of the drive component. The slider component is coupled to the housing and slidable along an elongate groove of the drive component such that movement of the slider component along the axis rotates the drive component within the housing.
Owner:AQUESYS INC
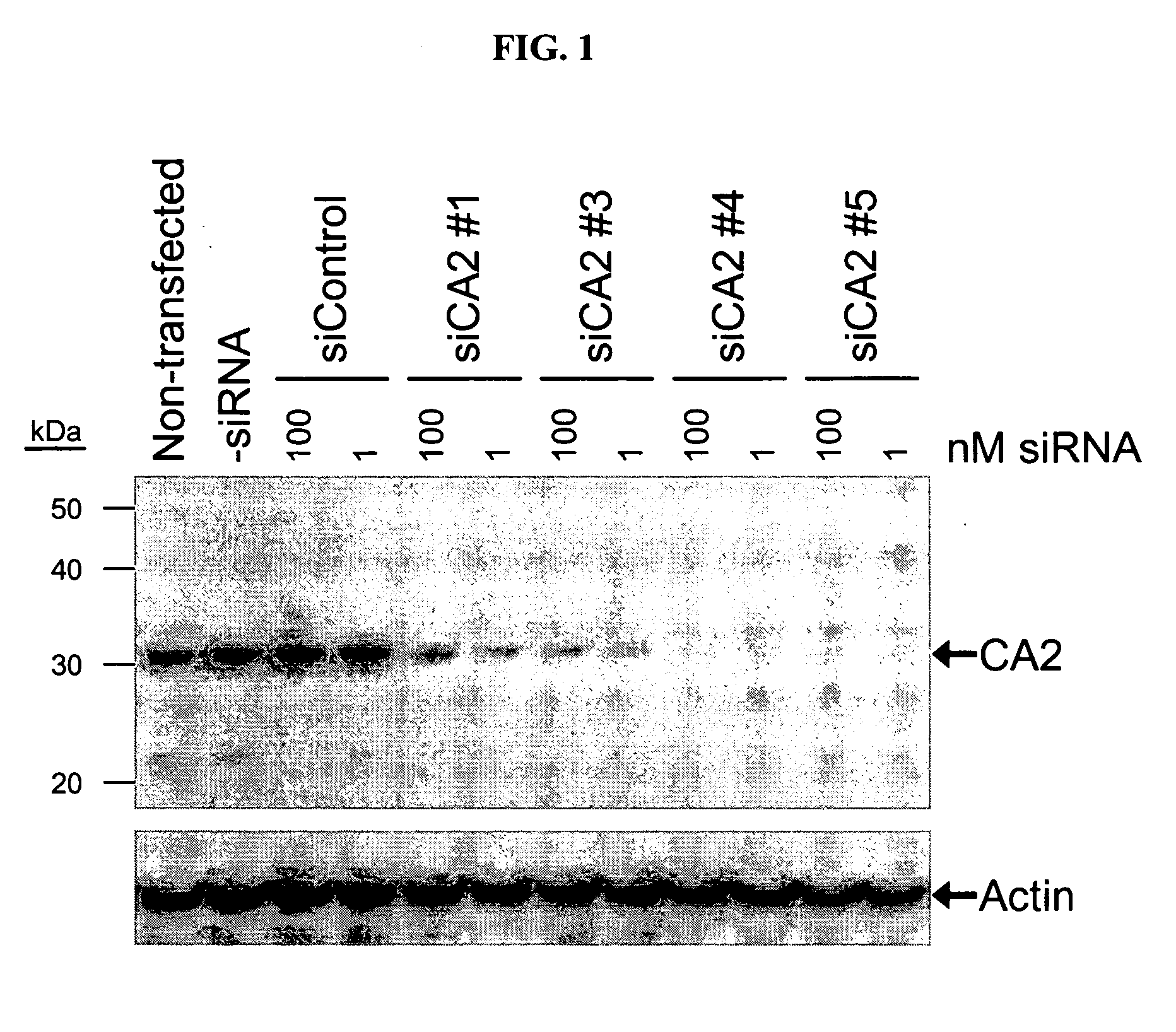
RNAi-mediated inhibition of ocular hypertension targets
InactiveUS20060172963A1Lower eye pressureOrganic active ingredientsSenses disorderIntra ocular pressureATPase
RNA interference is provided for inhibition of ocular hypertension target mRNA expression for lowering elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Ocular hypertension targets include carbonic anhydrase II, IV, and XII; β1- and β2 adrenergic receptors; acetylcholinesterase; Na+ / K+-ATPase; and Na—K-2Cl cotransporter. Ocular hypertension is treated by administering interfering RNAs of the present invention.
Owner:ARROWHEAD RES CORP +1
Combination of sulfonamide compound
ActiveUS20140018396A1Good effectReduce the impactBiocideOrganic chemistryIntra ocular pressureOphthalmology
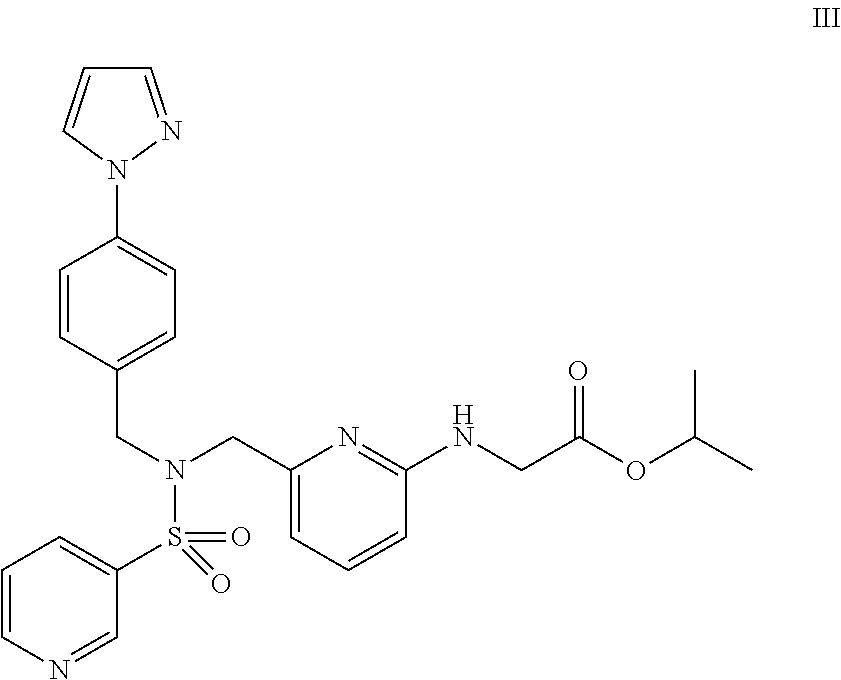
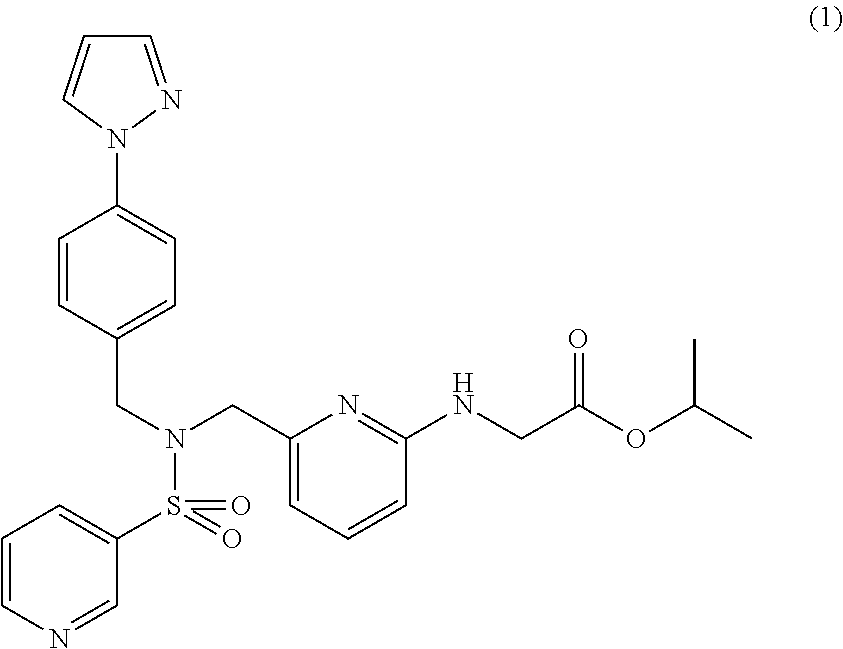
The object of the present invention is to discover a combination of preventive or therapeutic drugs for glaucoma or ocular hypertension, which is useful as a preventive or therapeutic agent for glaucoma or ocular hypertension. By combining isopropyl(6-{[4-(pyrazol-1-yl)benzyl](pyridin-3-ylsulfonyl)aminomethyl}pyridin-2-ylamino)acetate with other preventive or therapeutic drug for glaucoma or ocular hypertension, their intraocular pressure lowering effects are complemented and / or enhanced each other. As for the administration form, these drugs may be administered concomitantly or may be administered as a combination drug.
Owner:SANTEN PHARMA CO LTD
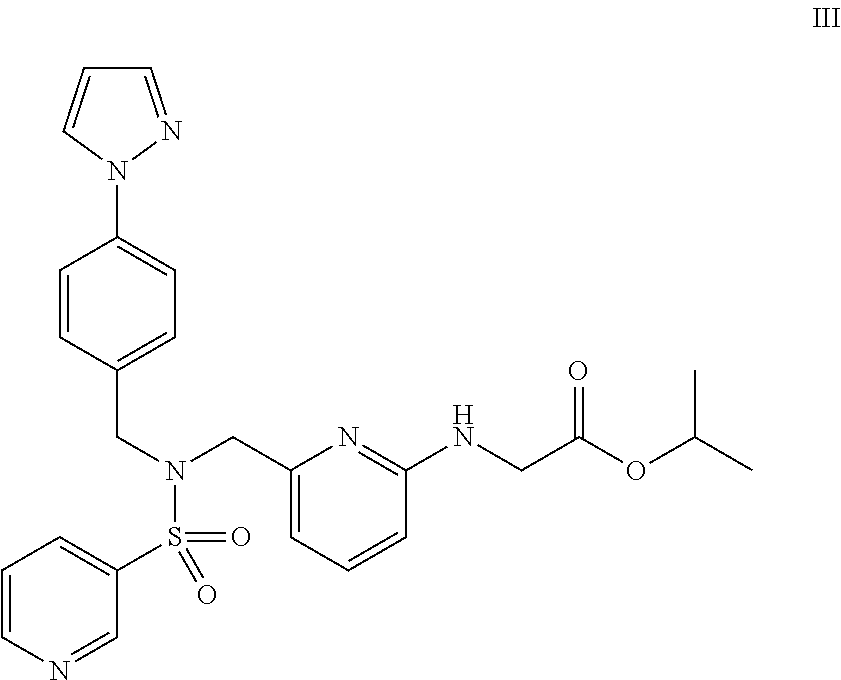
Combination of sulfonamide compound and tafluprost
InactiveUS20140018350A1Good effectReduce the impactBiocideOintment deliveryTherapy medicationBenzyl group
The object of the present invention is to discover a combination of preventive or therapeutic drugs for glaucoma or ocular hypertension, which is useful as a preventive or therapeutic agent for glaucoma or ocular hypertension. By combining isopropyl(6-{[4-(pyrazol-1-yl)benzyl](pyridin-3-ylsulfonyl)aminomethyl}pyridin-2-ylamino)acetate with tafluprost, their intraocular pressure lowering effects are complemented and / or enhanced each other. As for the administration form, these drugs may be administered concomitantly or may be administered as a combination drug.
Owner:SANTEN PHARMA CO LTD +1
Methods and compositions for the treatment of ocular disorders
The invention provides methods and compositions for the delivery of lipophilic drugs that are useful for the treatment of various ophthalmological diseases, disorders, and pathologies, including the treatment of age-related macular degeneration, diabetic retinopathy, diabetic macular edema, cancer, and glaucoma.
Owner:TARGEGEN
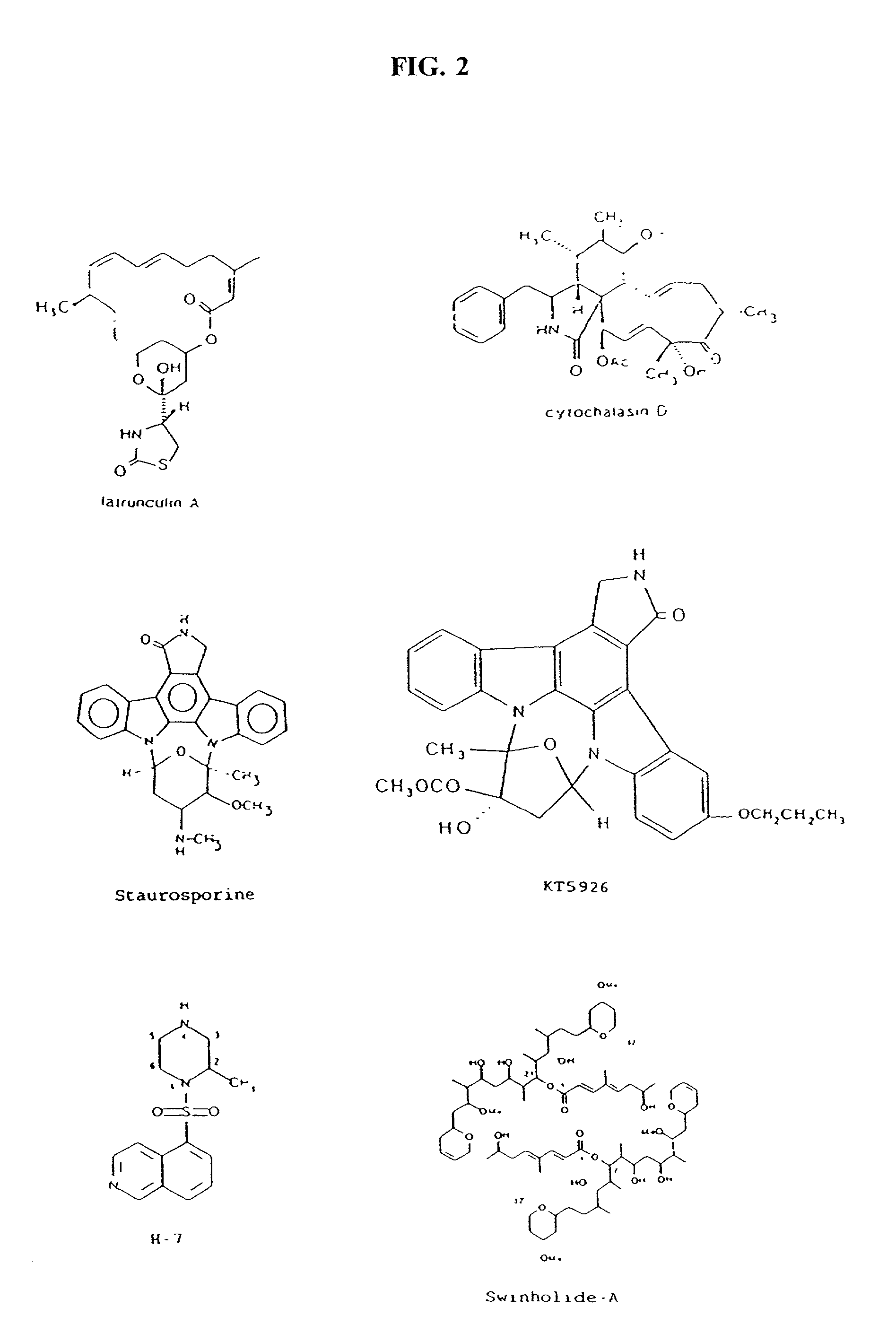
Cytoskeletal active agents for glaucoma therapy
InactiveUS20020045585A1Lower eye pressureImprove fluid flowBiocideSenses disorderActin cytoskeletonActive agent
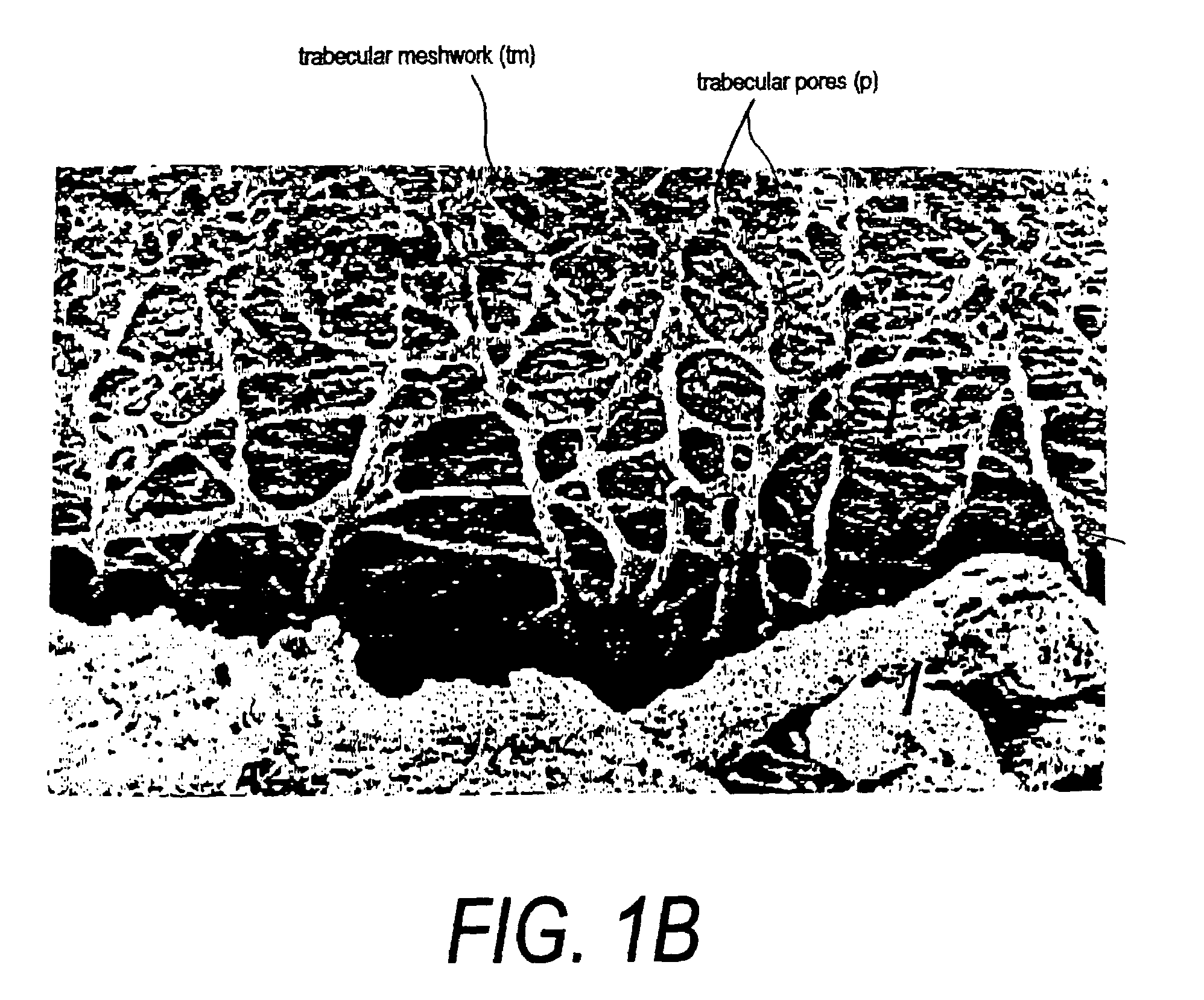
Methods for the treatment of glaucoma are provided by the present invention. The compounds described cause a perturbation of the actin cytoskeleton in the trabecular meshwork or the modulation of its interactions with the underlying membrane. Perturbation of the cytoskeleton and the associated adhesions reduces the resistance of the trabecular meshwork to fluid flow and thereby reduces intraocular pressure.
Owner:YEDA RES & DEV CO LTD +1
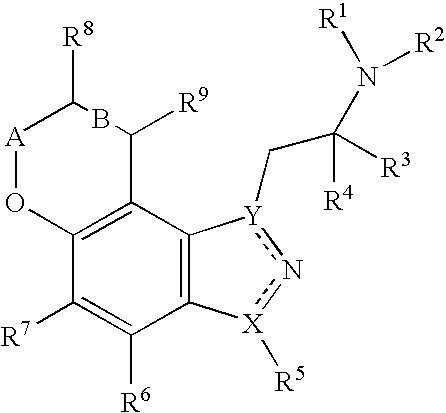
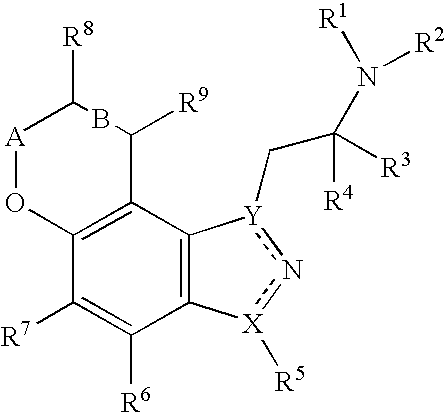
Pyranoindazoles and their use for the treatment of glaucoma
InactiveUS6696476B2Good chemical stabilityOrganic active ingredientsBiocideElevated intraocular pressureTreatment glaucoma
Pyranoindazoles are disclosed. Also disclosed are methods for the lowering and controlling of normal or elevated intraocular pressure as well as a method for the treatment of glaucoma using compositions containing one or more of the compounds of the present invention.
Owner:NOVARTIS AG
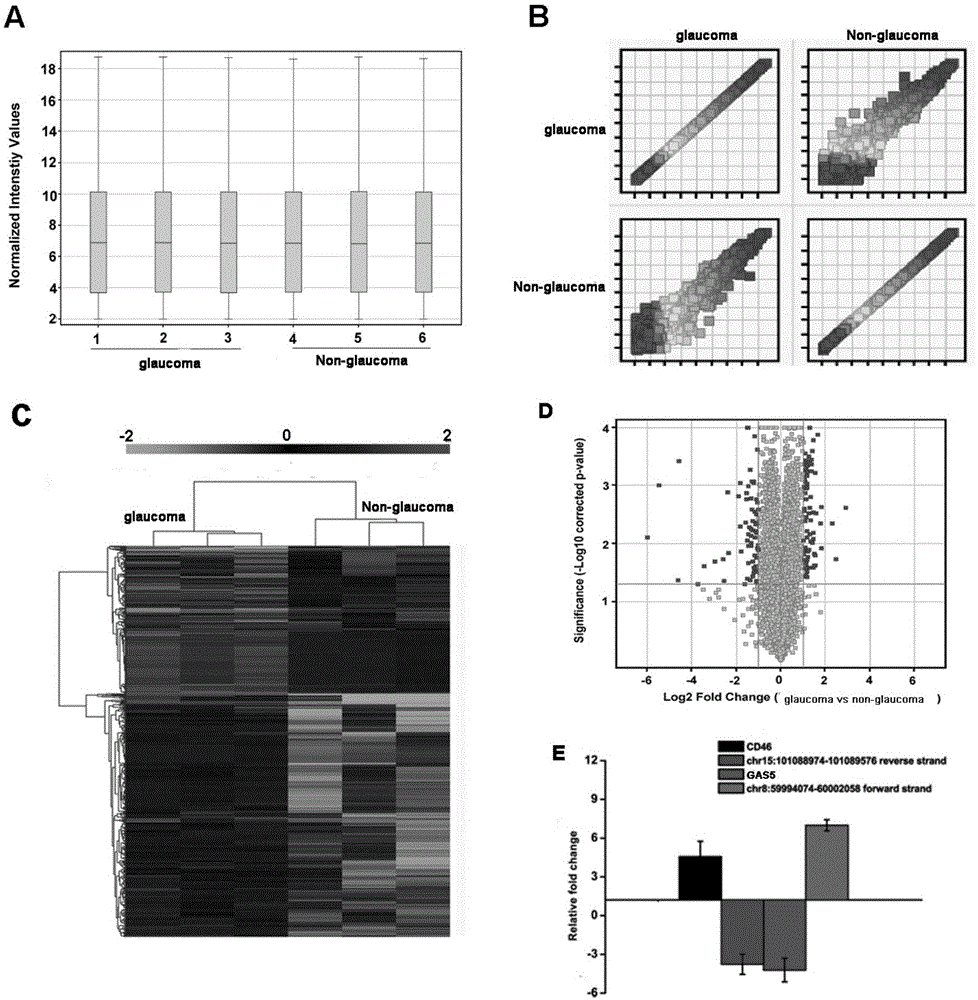
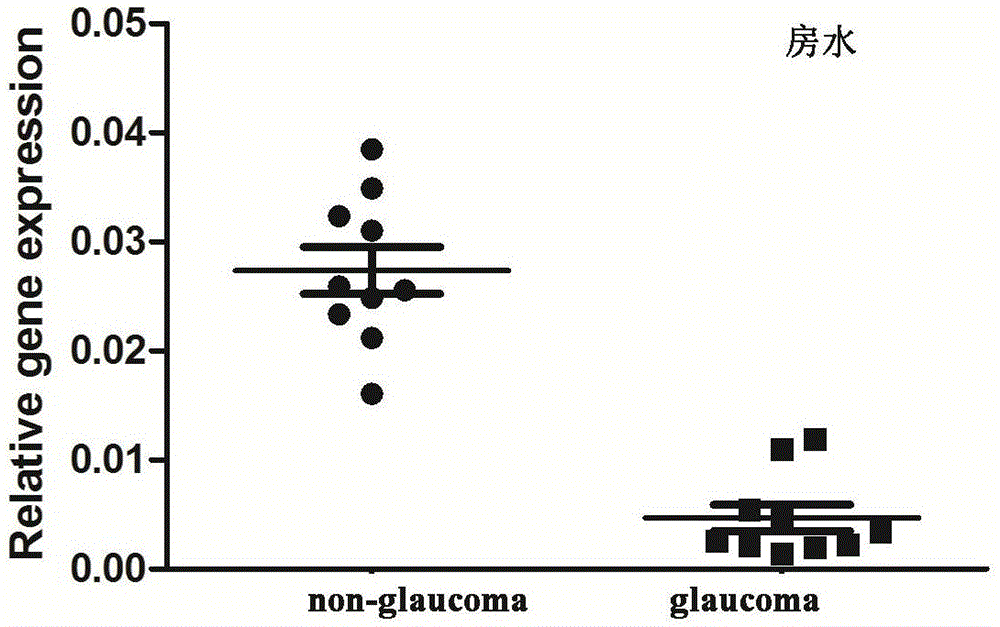
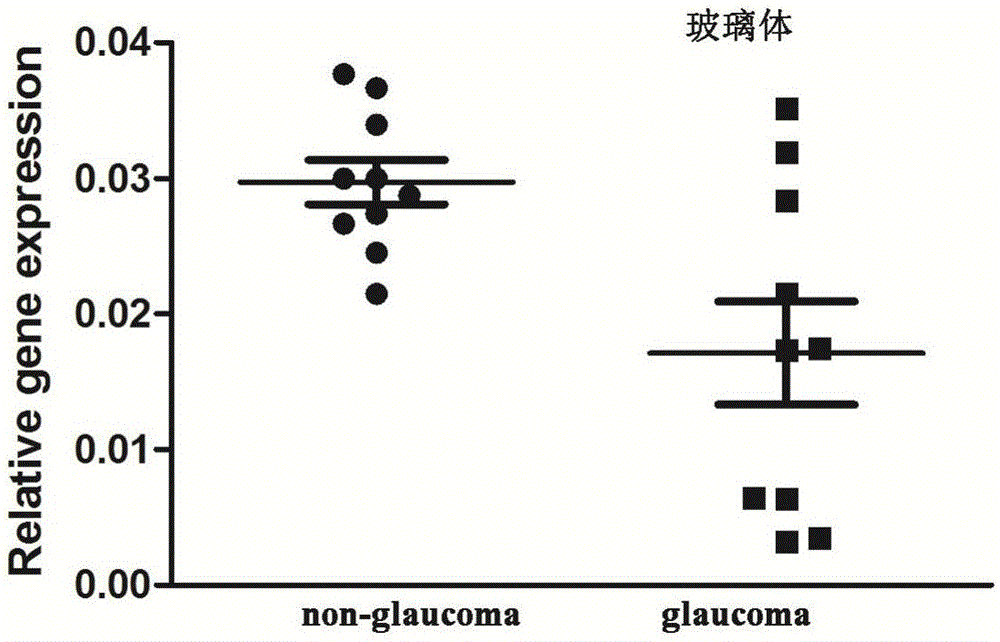
Application of LncRNA-GAS5 in preparing glaucoma diagnosis reagent
ActiveCN105002182AGood correlationReduce healingSenses disorderGenetic material ingredientsGlaucomaOcular disease
The invention discloses the application of LncRNA-GAS5 in preparing a glaucoma diagnosis reagent and a glaucoma lesion diagnosis reagent kit. Through high-throughput sequencing and quantitative PCR validation, it is found that the LncRNA-GAS5 has a significant correlation with the occurrence of glaucoma. The LncRNA-GAS5 can be used for screening clinical glaucoma patients and providing a theoretical basis for early intervention in glaucoma.
Owner:南京医科大学眼科医院
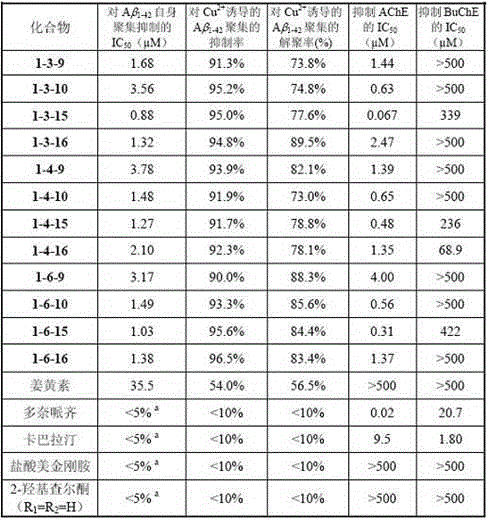
2-hydroxyl chalcone compound as well as preparation method and purpose thereof
ActiveCN105481706AEnhanced inhibitory effectStrong inhibitory activitySenses disorderNervous disorderHuntingtons choreaNeuro-degenerative disease
The invention discloses a novel 2-hydroxyl chalcone compound (I), as well as pharmacy acceptable salt, preparation method, medical composition, and purpose thereof in preparing medicines for treating and / or preventing neurodegenerative diseases including but not limited to vascular dementia, Alzheimer's diseases, Parkinson's diseases, Huntington's diseases, dementia relevant to HIV, multiple sclerosis, amyotrophic lateral sclerosis, neuropathic pain, glaucoma and the like (As shown in the description.).
Owner:SICHUAN UNIV
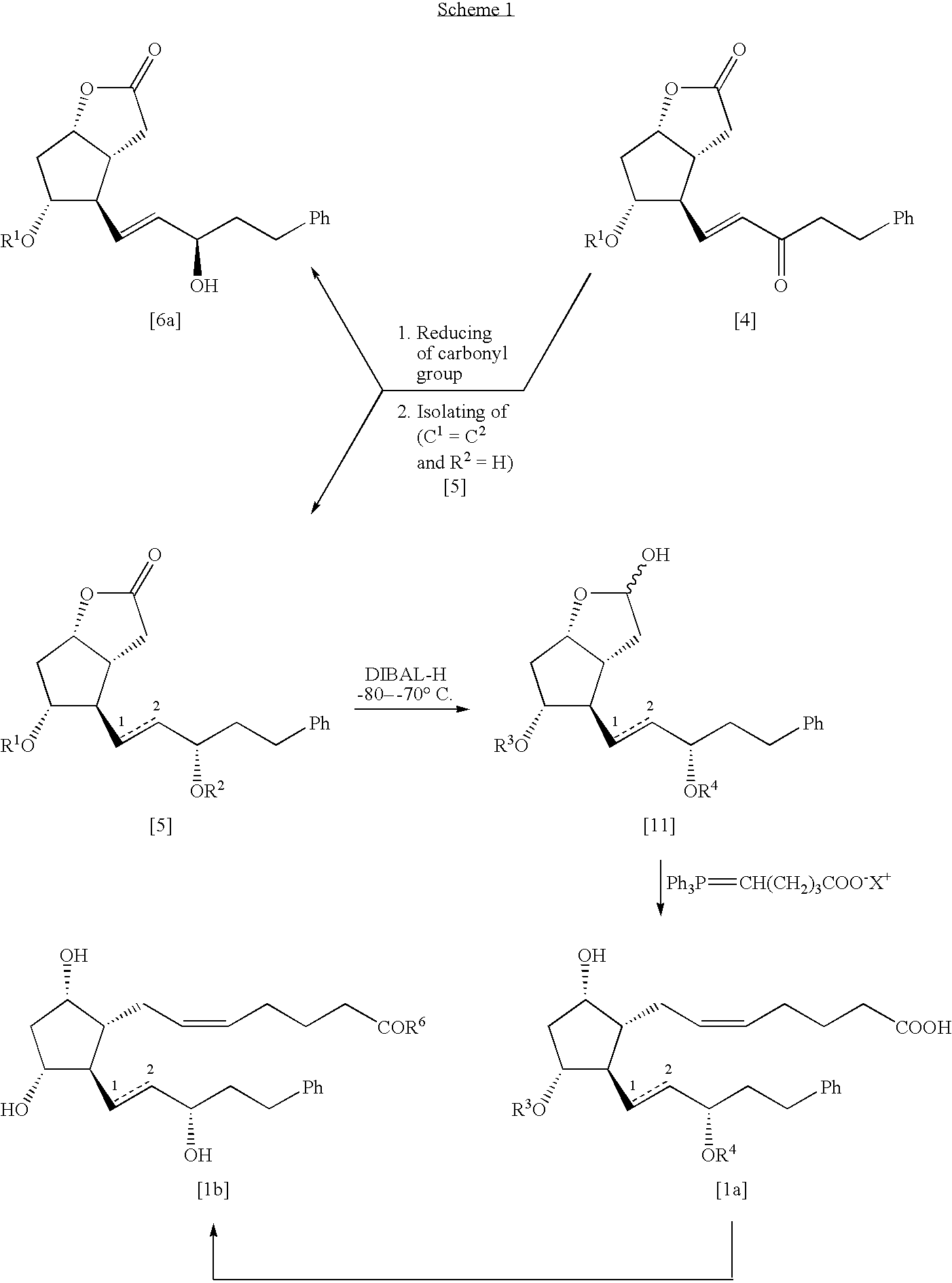
Process for the preparation of 17-phenyl-18,19,20-thinor-pgf 2a and its derivatives
InactiveUS7157590B2High yieldDesired purityOrganic compound preparationOrganic chemistry methodsLatanoprostOphthalmology
The present invention provides a new and effective process for the synthesis of 17-phenyl-18,19,20-trinor-PGF2α and its derivatives, including the anti-glaucoma drugs Bimatoprost and Latanoprost. The benefit of the present invention rises inter alia from the fact that a major intermediate involved in the synthesis of the above compounds may be isolated from a mixture containing also an undesired isomer, by crystallization. In addition, the undesired isomer may be oxidized to give the starting compound, which is then recycled.
Owner:FINETECH PHARMA
Devices and Methods Useable for Treatment of Glaucoma and Other Surgical Prcedures
ActiveUS20160106589A1Avoiding significant and irreparable damageEye surgeryDisinfectionSurgical departmentTissue protection
A device and method for cutting or ablating tissue in a human or veterinary patient includes an elongate probe having a distal end, a tissue cutting or ablating apparatus located adjacent within the distal end, and a tissue protector extending from the distal end. The protector generally has a first side and a second side and the tissue cutting or ablating apparatus is located adjacent to the first side thereof. The distal end is structured to be advanceable into tissue or otherwise placed and positioned within the patient's body such that tissue adjacent to the first side of the protector is cut away or ablated by the tissue cutting or ablation apparatus while tissue that is adjacent to the second side of the protector is not substantially damaged by the tissue cutting or ablating apparatus.
Owner:MICROSURGICAL TECH INC
Flavone alkylamine compounds as well as preparation method and application thereof
The invention discloses a type of novel flavone alkylamine compounds with the formula (I) and pharmaceutically acceptable salts, a preparation method, a pharmaceutical composition and application in preparation of medicines for treating and / or preventiung neurodegeneration related diseases. The diseases comprise but are not limited to vascular dementia, Alzheimer's disease, Parkinson's disease, Huntington's disease, HIV related dementia, multiple sclerosis, progressive lateral sclerosis, neuropathic pain, glaucoma and other neurodegeneration diseases.
Owner:SICHUAN UNIV
Pharmaceutical formulations comprising a pyridylaminoacetic acid compound
ActiveUS20150196541A1Good prevention effectGood treatment effectBiocideSenses disorderOcular tensionOphthalmology
Provided is a pharmaceutical preparation for treatment or prevention of glaucoma or ocular hypertension, comprising 0.0003 to 0.01% (w / v) of isopropyl(6-{[4-(pyrazol-1-yl)benzyl](pyridin-3-ylsulfonyl)aminomethyl}pyridin-2-ylamino)acetate, or a salt thereof.
Owner:SANTEN PHARMA CO LTD
Method and apparatus for treating an ocular disorder
Embodiments of the claimed invention are directed to the treatment of glaucoma (or conditions of elevated intraocular pressure) using a novel ab interno trabeculotomy procedure that uses a flexible device. At least one advantage of the present method is that it does not require a conjunctival or scleral incision, which in turn improves patient recovery time and healing.
Owner:NOVA EYE INC
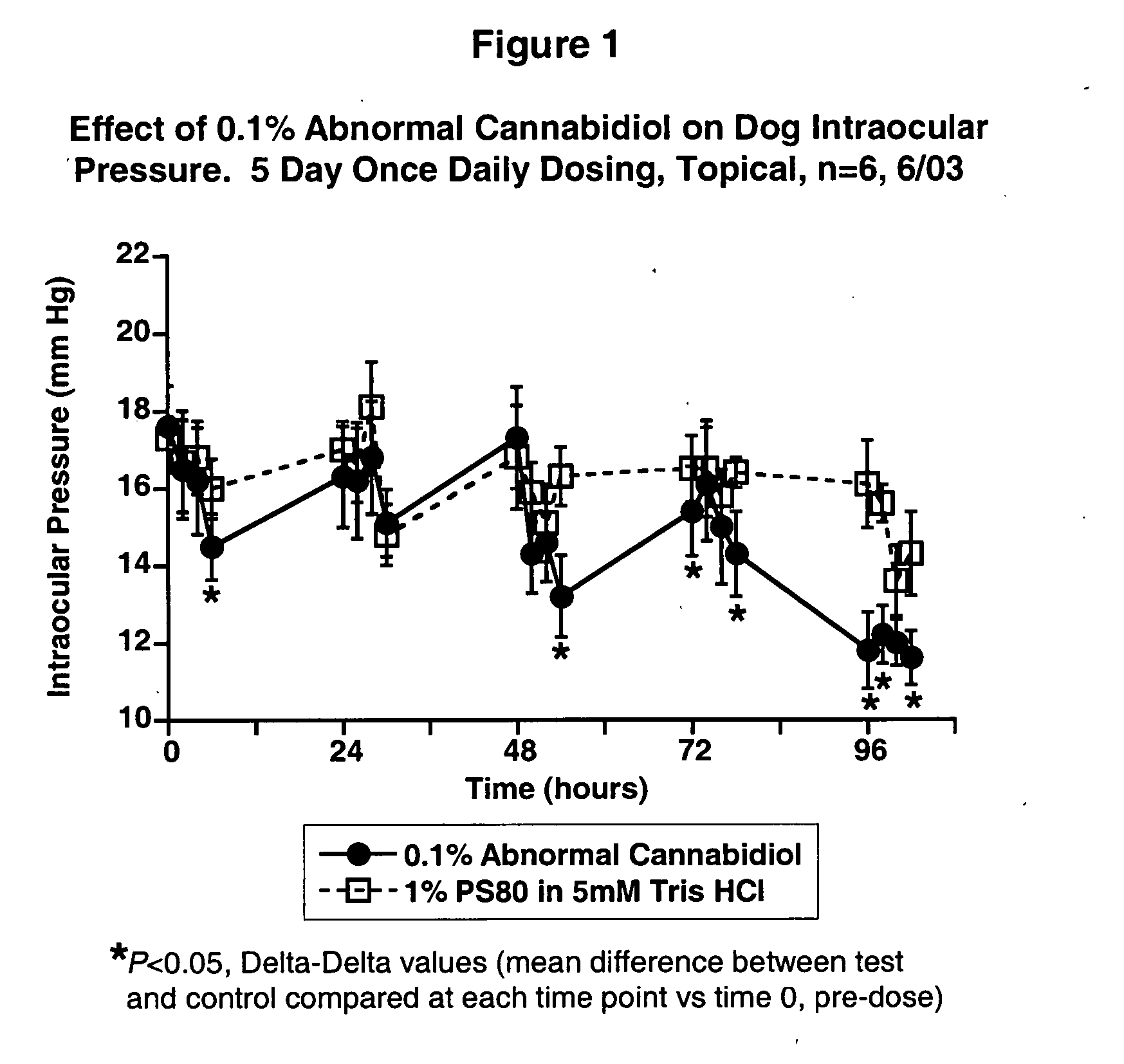
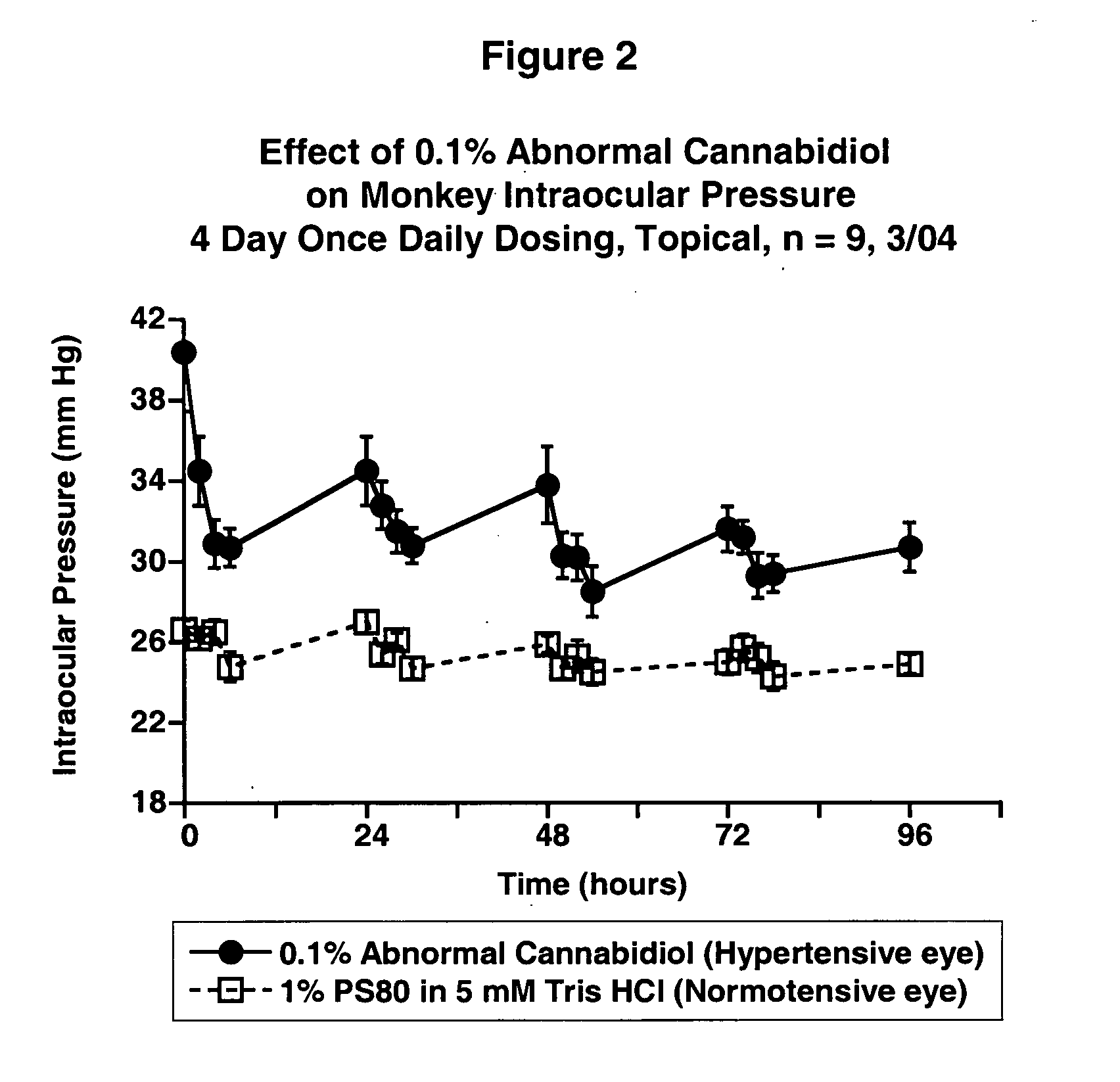
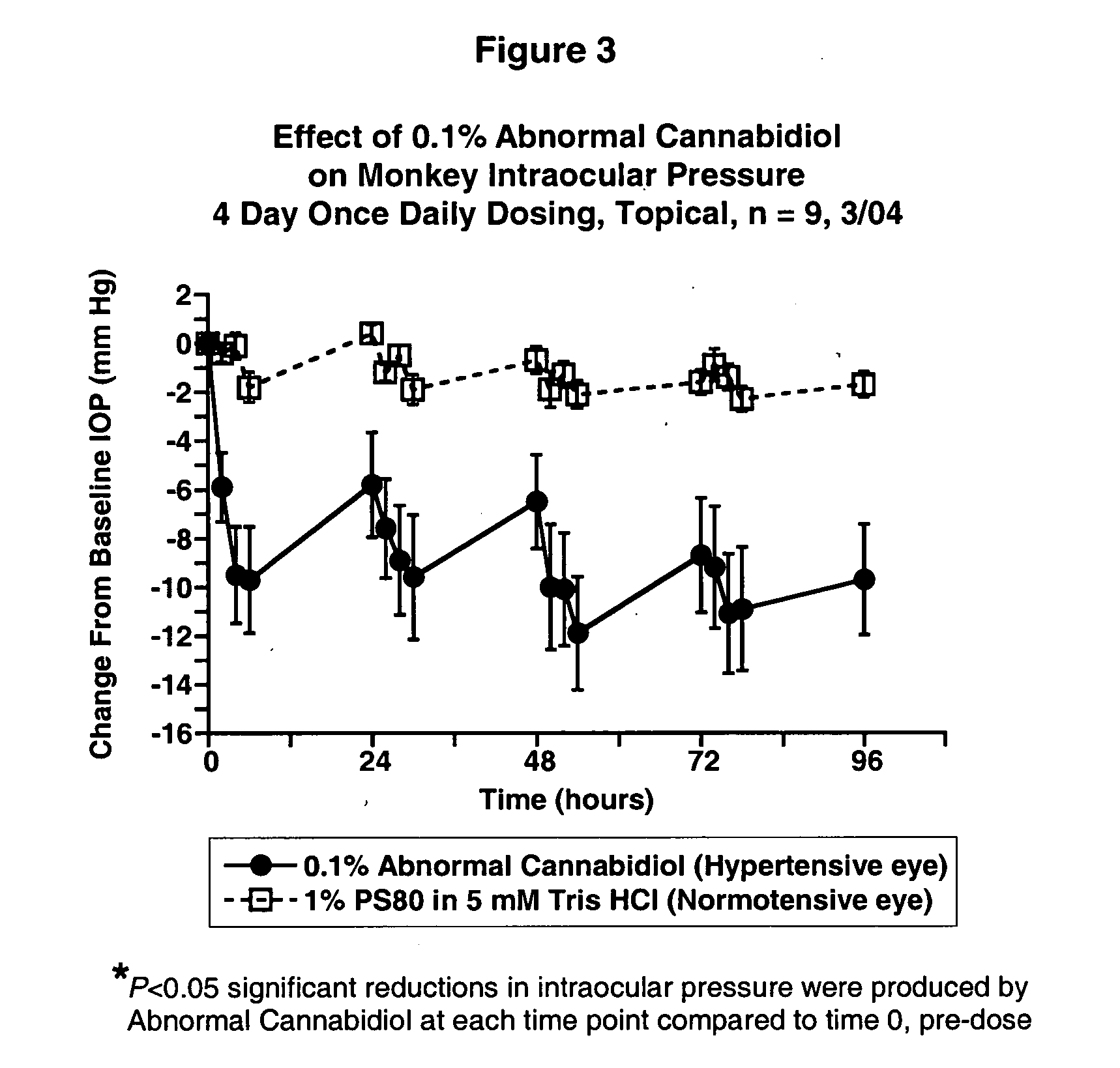
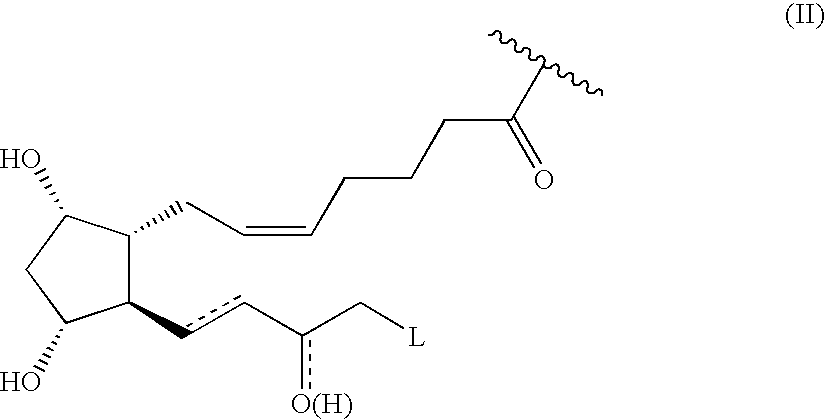
Abnormal cannabidiols as agents for lowering intraocular pressure
InactiveUS20050282902A1Lower intraocular pressureBiocideElcosanoid active ingredientsDrugAdrenergic agonist
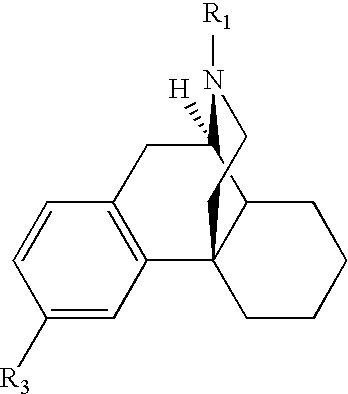
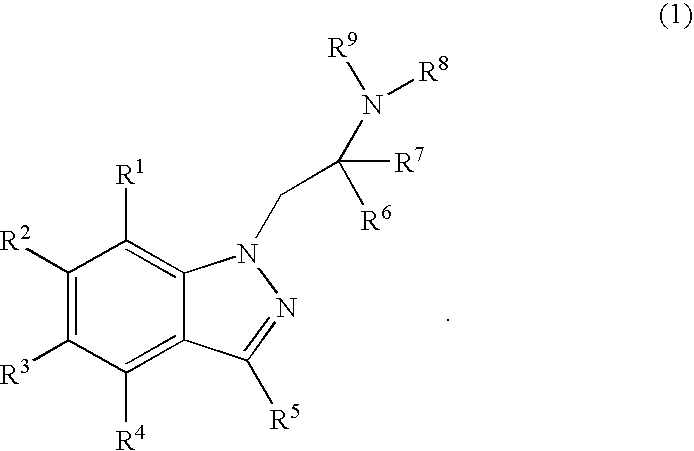
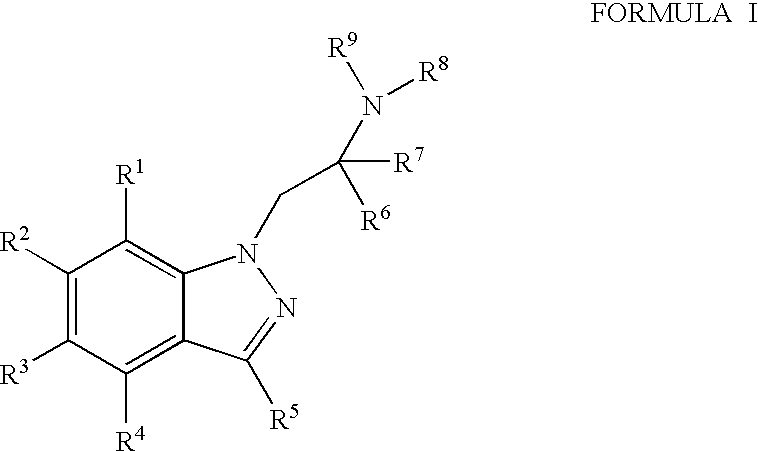
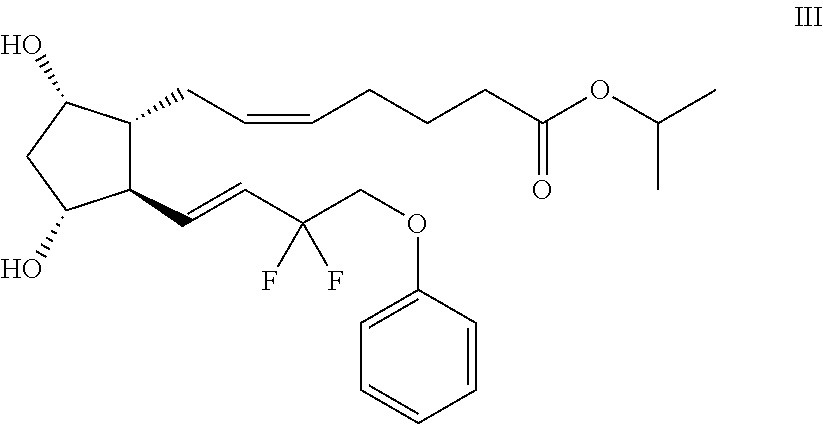
The invention relates to the use of Abnormal Cannabidiols in a combination with a drug selected from the group consisting of β-blockers, adrenergic agonists, carbonic anhydrase inhibitors, cholinergic agonists, chlolinesterase inhibitors, glutamate antagonists, prostamides and prostaglandins and the like, or pharmaceutically acceptable salts or prodrugs thereof as potent ocular hypotensives. Said combinations are particularly suitable for the management of glaucoma. In particular said Abnoral Cannibidiols are represented by formula I or formula II or formula III
Owner:ALLERGAN INC
Prostaglandin derivatives
ActiveUS7273946B2Enhance the imageEliminate orBiocideSenses disorderIntra ocular pressureTolerability
Prostaglandin nitroderivatives having improved pharmacological activity and enhanced tolerability are described. They can be employed for the treatment of glaucoma and ocular hypertension.
Owner:NICOX SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com