Application of LncRNA-GAS5 in preparing glaucoma diagnosis reagent
A technology of diagnostic reagents and diagnostic kits, applied in the medical field to achieve the effect of reducing treatment and medical expenses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
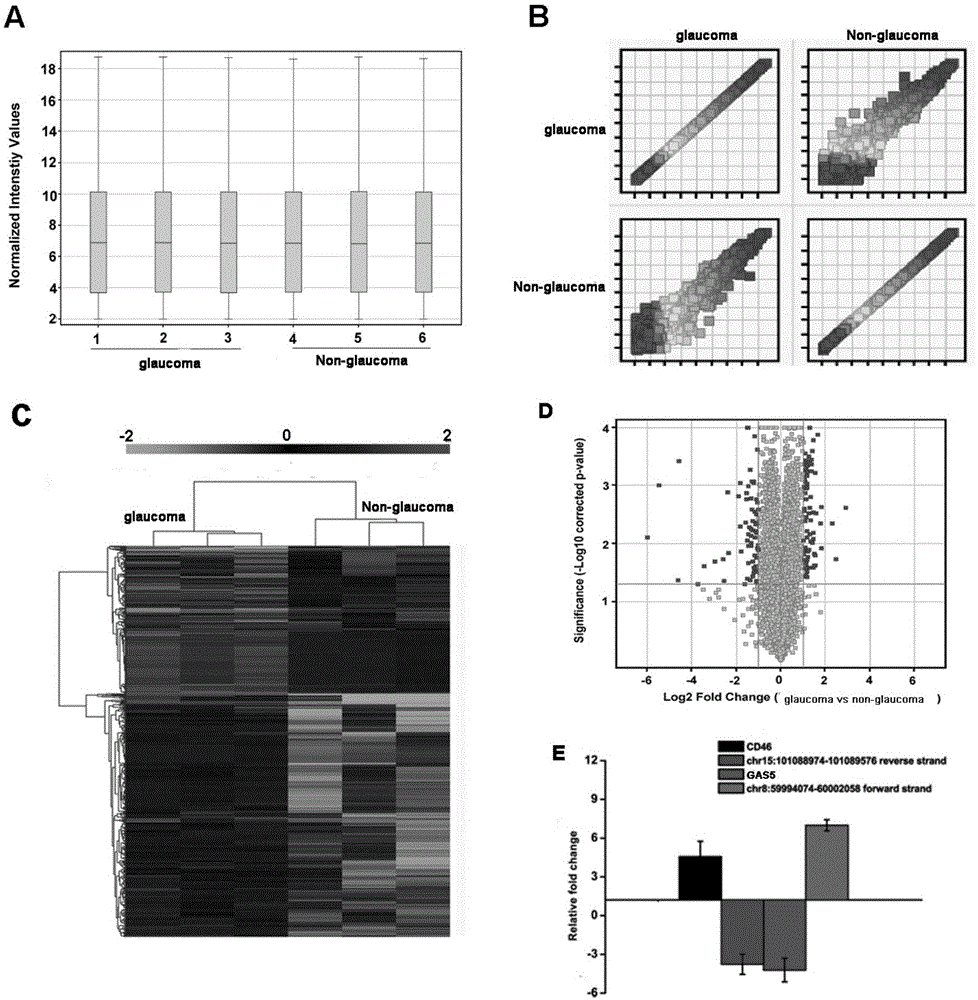
[0053] Example 1 Verification of the correlation between LncRNA-GAS5 and glaucoma lesions
[0054] (1) lncRNA microarray analysis and screening of lncRNAs related to glaucoma diseases and verification
[0055] The first step: sample preparation: the pathological tissue trabecular meshwork (experimental group, n=21) and normal traumatic human trabecular meshwork (control group, n=21) were collected during glaucoma surgery, and TRIzol (Invitrogen) reagent was used for total RNA Extract and store at -80°C for later use.
[0056] Step 2: Differentially expressed lncRNA screening:
[0057] Using the lncRNA expression profile chip of Aglient Company in the United States, the lncRNA related to the occurrence of glaucoma disease was analyzed; the specific steps of the analysis were: using labeling enzyme to label the lncRNA with the fluorescent group, and obtain the fluorescent probe for hybridization with the chip, and use MAUI under the labeling conditions Hybridization instrument...
Embodiment 2
[0061] Embodiment 2 prepares kit of the present invention
[0062] The sequence of LncRNA-GAS5 is shown as SEQ ID: NO: 1, its specific quantitative PCR upstream and downstream primers and internal reference GAPDH and / or Beta-tubulin quantitative PCR upstream and downstream primers are designed by Primer 5, and Invitrogen is responsible for primer synthesis , the purity is PAGE grade, and the synthesized primers use DEPC H 2 O was dissolved to a total concentration of 10 μM.
[0063] Prepare a kit comprising the following components:
[0064] Extraction system
[0065] 1) Trizol reagent, 1 tube, 2000 μL / tube;
[0066] 2) Chloroform, 1 tube, 500 μL / tube;
[0067] 3) Absolute ethanol, 1 tube, 8000 μL / tube;
[0068] 4) DEPC ddH 2 O, 1 tube, 1000 μL / tube;
[0069] 5) ddH2O, 1 tube, 2000 μL / tube;
[0070] 6) Isopropanol, 8000 μL / tube;
[0071] reverse transcription system
[0072] 1) Total RNA reverse transcription primers (including Oligo dT and Random6mers), 1 tube, conc...
Embodiment 3
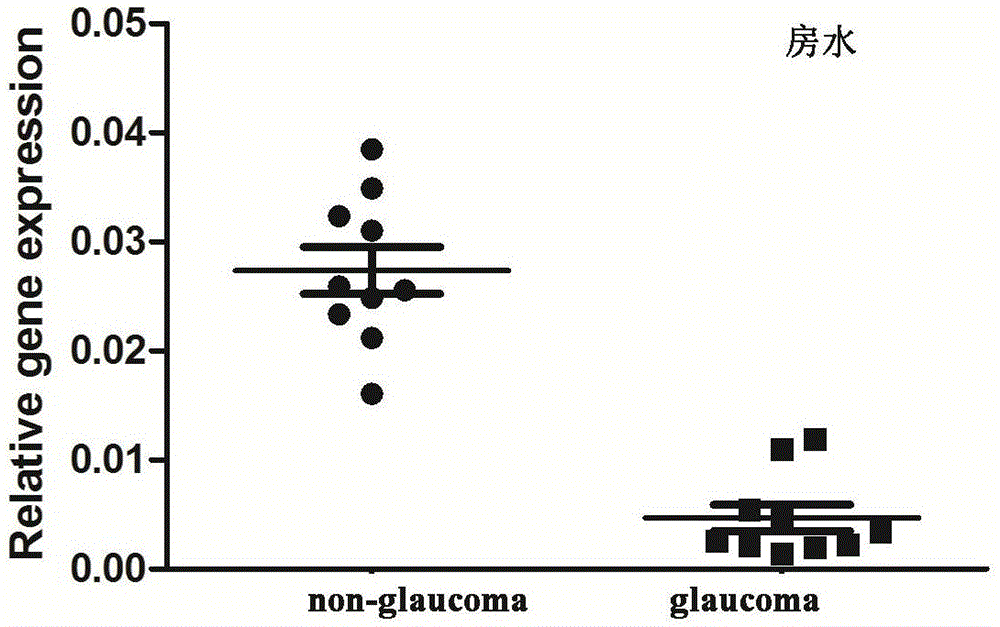
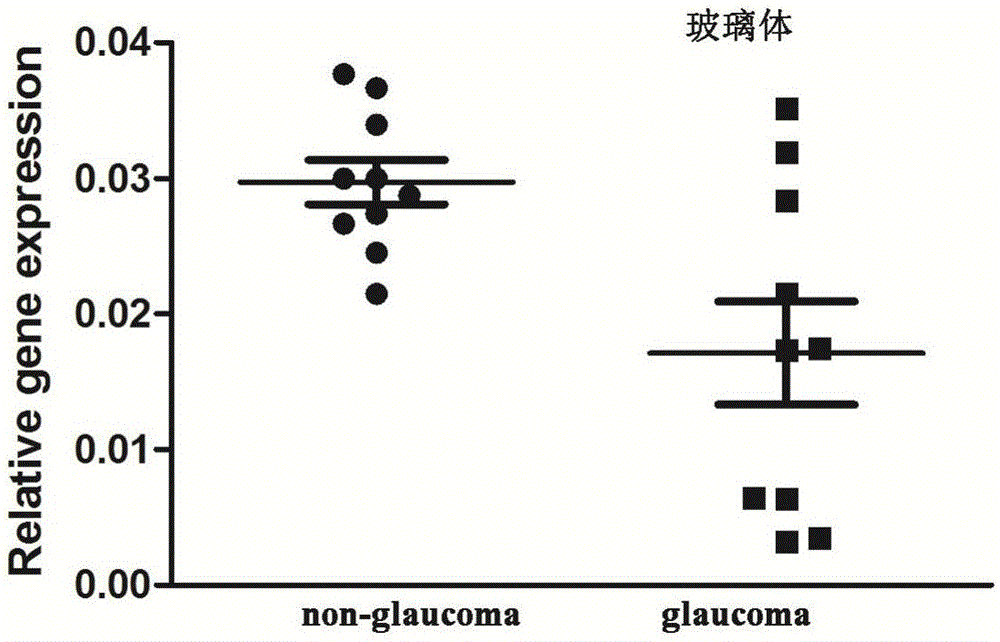
[0086] Example 3 Detection of the expression of LncRNA-GAS5 in aqueous humor, vitreous, blood cells and plasma
[0087] Blood sample: 5 mL of venous blood was drawn on an empty stomach in the morning, added to a test tube containing 2% sodium citrate, and centrifuged to obtain plasma and blood cell samples at 4°C, 12,000 rpm, 10 min.
[0088] Aqueous humor specimens: before opening the anterior chamber or performing eyeball incision, draw 0.1mL of anterior humor directly with a 1.0mL sterile disposable plastic syringe, and transfer it into a sterilized 1.0mL effendorf tube for storage at low temperature.
[0089] Vitreous sample: When performing standard three-channel vitreous cutting, without turning on the water inlet switch, directly extract 0.3 mL of vitreous body from the opening of the cutting head, and transfer it into a sterilized 1.0 mL effendorf test tube for low temperature storage.
[0090] RNA extraction from aqueous humor, vitreous, serum and plasma samples
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com