2-hydroxyl chalcone compound as well as preparation method and purpose thereof
A hydroxychalcone and compound technology, which is applied to a class of 2-hydroxychalcone compounds, the fields of preparation and use thereof, and can solve the problems of unsatisfactory depolymerization activity, poor curative effect, weak inhibitory effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
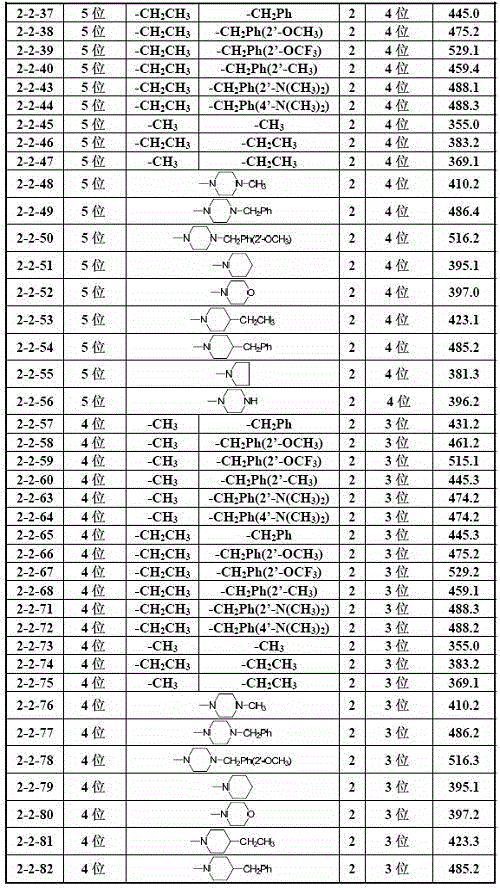
Embodiment 12
[0034] Example 12-hydroxyl ketones (i) preparation method
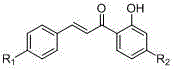
[0035] Add the corresponding 2-hydroxybenzhenylene compounds (2), 3.0mmol corresponding benzaldehyde compounds (1) and 30ml ethanol in the reaction bottle to the reaction bottle. After mixing well, add 30%KOH aquatic solution 8.0 mmol to the temperature.Return stirring reaction 3.0 to 24.0 hours (tracked with TLC); after the reaction process is completed, it is cooled to room temperature, and the pH is most acidic with 10%hydrochloride solution, and then the pH is used to regulate the pH to the weak alkali with saturated sodium bicarbonate aquatic solution.Sexual, reducing degradation, reducing ethanol, adding 80ml of removal water to the residual liquid, three times with 240ml dichloromethane, washed three times after the organic layer merged with saturated sodium chloride solution, dried after drying sodium sulfateIn addition to the solvent, the residue is purified by the column layer (elute: dichloromethane: acetone = ...
Embodiment 22
[0073] Example 22-hydroxyl ketones (i) and acid-forming preparation method
[0074] Add 2-hydroxyl ketones (i) 2.0 mmol and acetone 50ml in the reaction bottle according to the above-mentioned Example 1, and add 8.0 mmol to the corresponding acid after stirring.By room temperature, decompress the solvents, residual substances with acetone, and the solids filtered out of the solid, that is, the salt of 2-hydroxyl ketones (i). 1 HNMR and ESI-MS are confirmed.
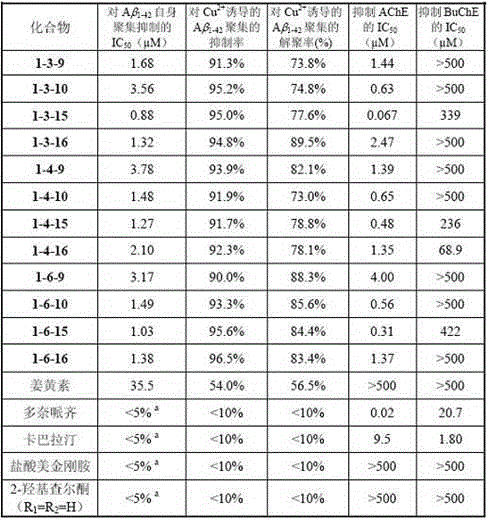
Embodiment 3
[0075] Example 3-biological activity screening results of 2-hydroxyl ketones (i)
[0076]
[0077] a The suppression rate at a concentration of 25.0 μm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com