Endovascular device with membrane
a technology of endothelial cells and endothelial cells, which is applied in the field of endothelial cells, can solve the problems of restricted blood flow, ischemia or necrosis, occlusion of coronary arteries, etc., and achieve the effects of promoting thrombosis, enhancing endothelial cell migration and tissue in-growth, and reducing blood flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
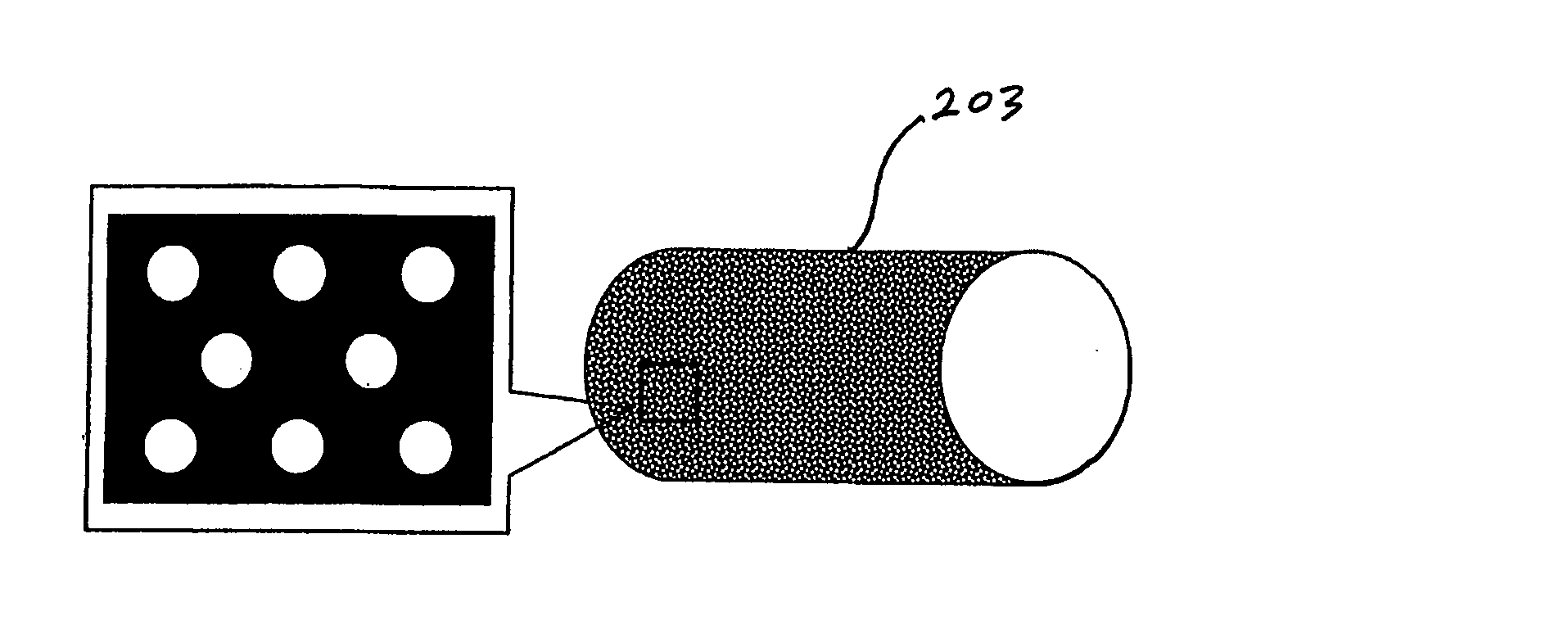
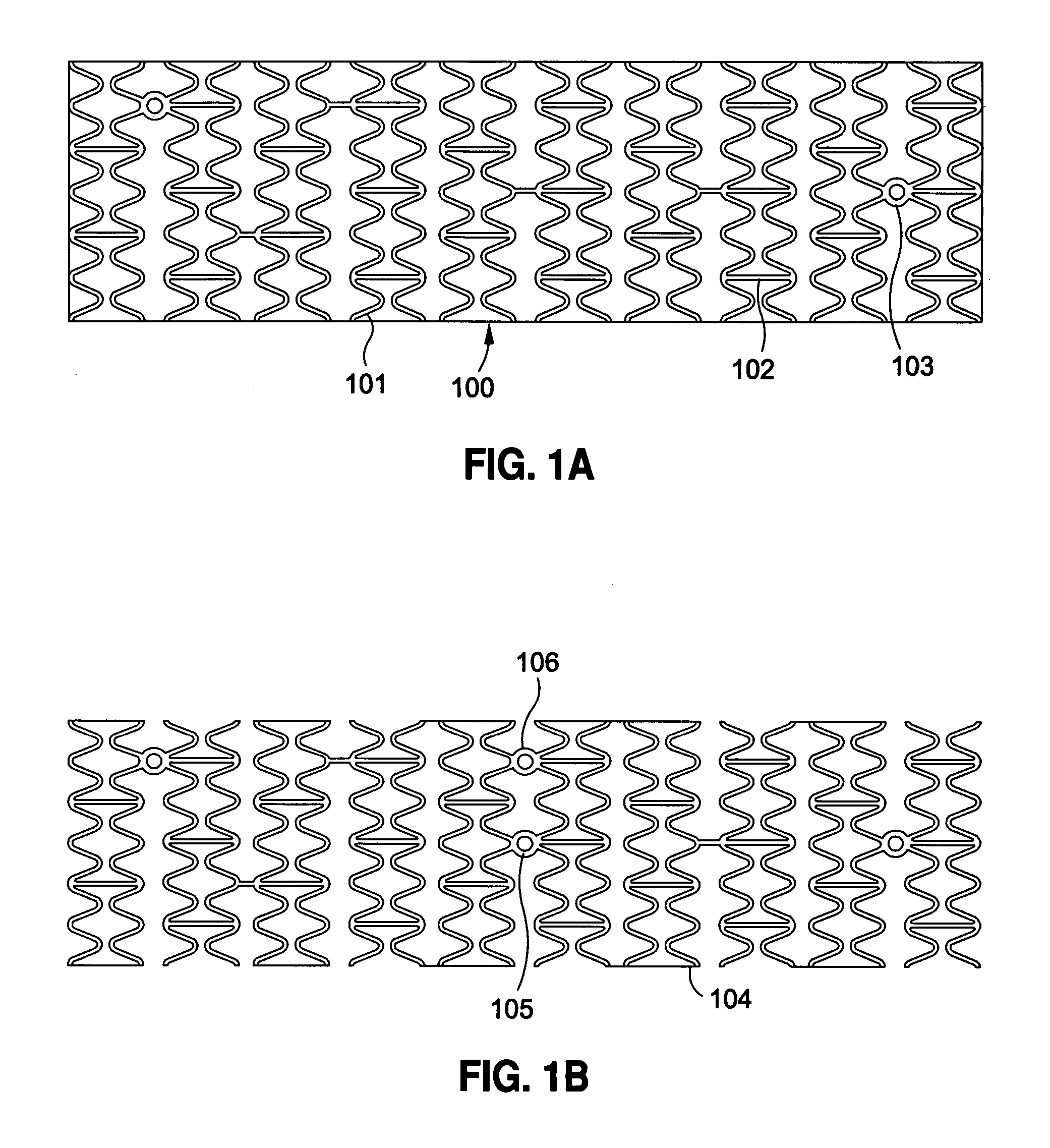
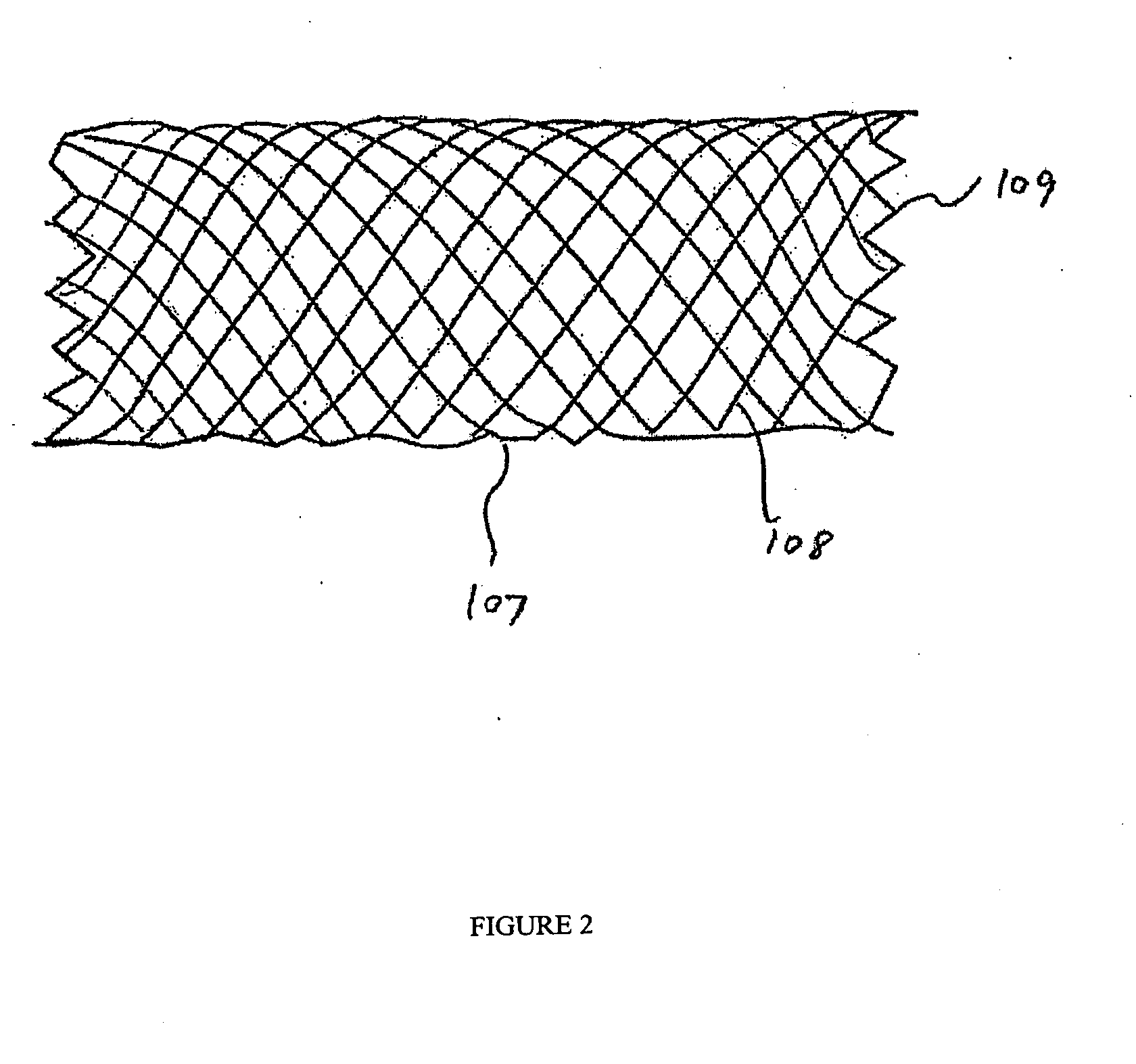
[0120] Implantable medical devices include physical structures for delivering drugs or reagents to desired sites within the endovascular system of a human body. These devices may take up diversified shapes and configurations depending upon specific applications. Common implantable medical devices include stents, vena cava filters, grafts and aneurysm coils.
[0121] The endovascular system of a human body includes blood vessels, cerebral circulation system, tracheo-bronchial system, the biliary hepatic system, the esophageal bowel system, and the urinary tract system. Although exemplary stents implantable in blood vessels are described, they are applicable to the remaining endovascular system. Embodiments of the invention, some of which are described herein are readily adaptable for use in the repair of a variety of vessels, including but not limited to, treatment or repair in cases of aneurysm, ischemic stroke, carotid artery stenosis, saphenous vein graft, small vessel stenosis, or ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com