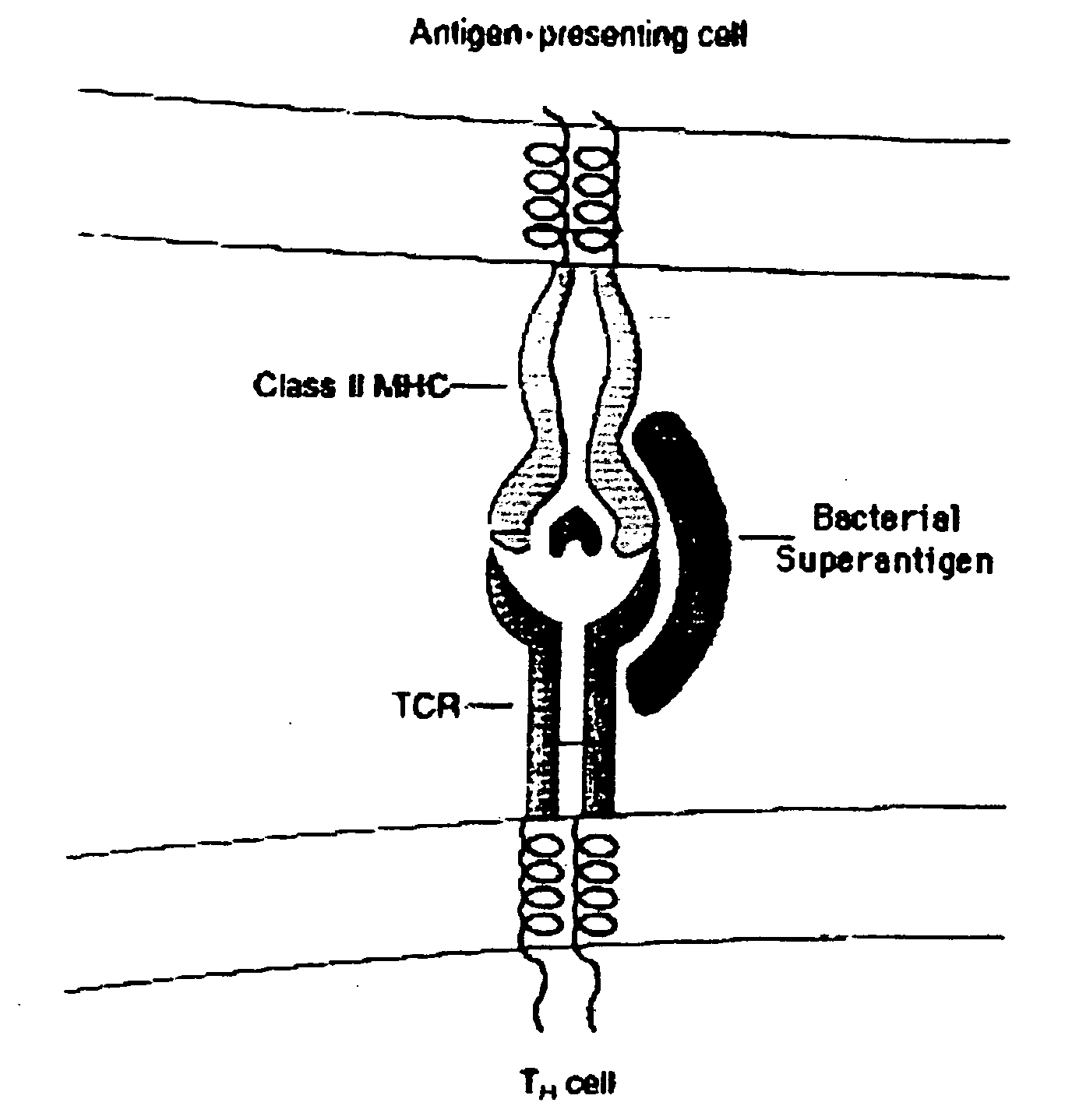
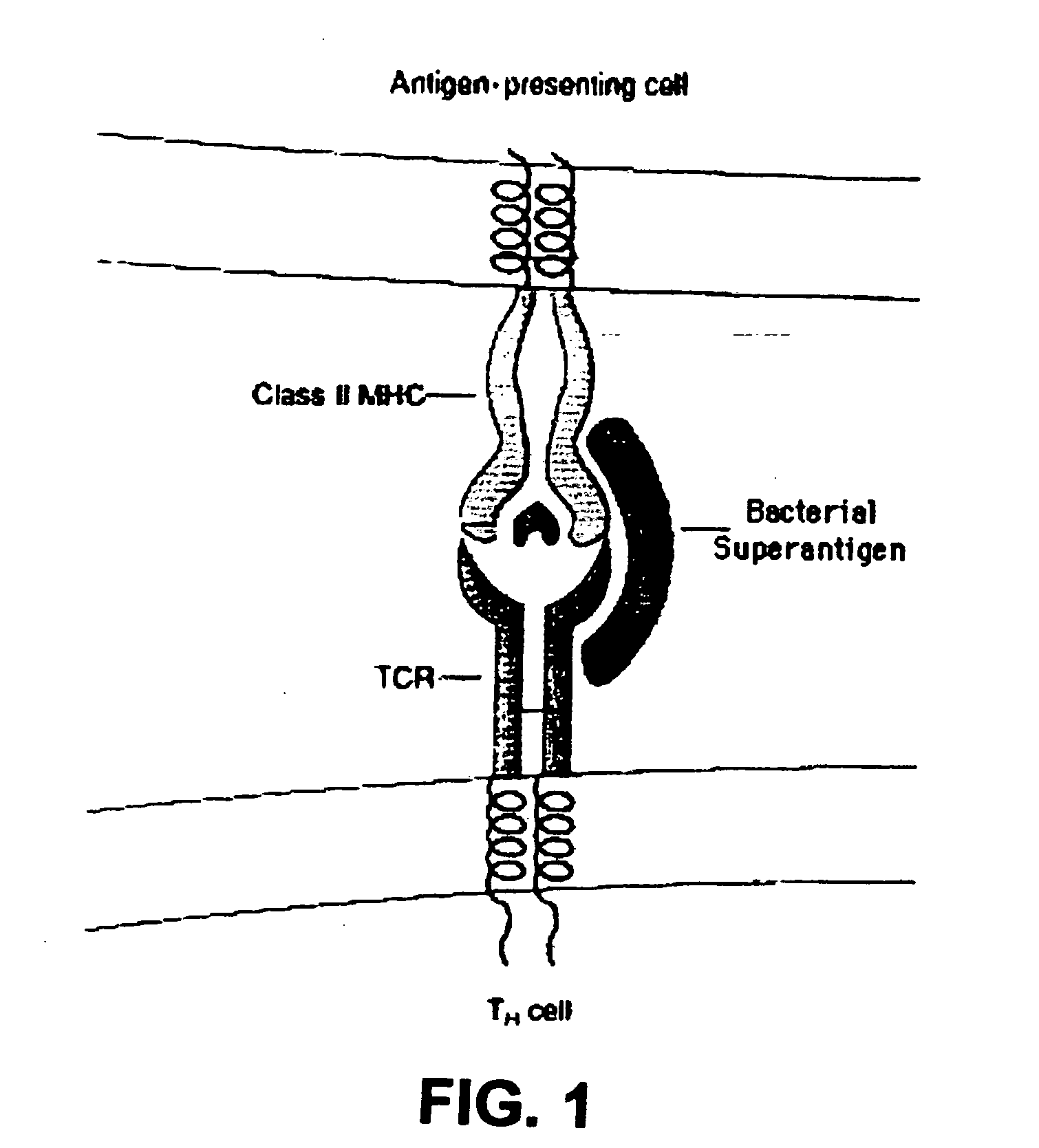
Method of passive immunization
a passive immunization and technology of peptides, applied in the field of peptides, can solve the problems of loss of mitogenic activity, high mortality of both diseases, skin loss,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0132]Peptides whose sequences are based on the two highly conserved regions of the staphylococcal and streptococcal pyrogenic exotoxins described herein were constructed. The sequences were based on alignments of the streptococcal pyrogenic exotoxins with the staphylococcal enterotoxins, and the amino acids used in positions with possible degeneracy were the amino acids most frequently found in these positions. Three of the peptides were then catenated and polymerized to produce peptides of greater than 8000 daltons (i.e., peptides 6343, 6345 and 6348, described below). As described further below, peptide 6348 was used to immunize rabbits, which produced high titer antibodies to this peptide. These antibodies were tested for the ability to recognize the streptococcal and staphylococcal pyrogenic exotoxins. Immunological assays (immunoblots) revealed that these antibodies recognized regions common to all the pyrogenic exotoxins. These antibodies were also tested for the ability to n...
example 2
[0150]PBMCs were isolated via Ficoll-Hypaque Solution. The appropriate concentration of nonpolymeric peptide and 2×105 cells in 200 μL of RPMI solution was plated in each well. The cells were Incubated for one hour at 37 degrees Centigrade, with mild agitation every 15 minutes. After one hour, 2 μg of SEB was added in each well. The PBMCs were incubated for 72 hours and the results were measured via tritiated thymidine incorporation. The cells were collected and read on a beta counter. The results are shown in FIG. 7. Note the dose-response inhibition of blastogenesis demonstrated in FIG. 7A. Peptide 6343 (i.e., CMYGGVTEGEGN, SEQ ID NO:3) (FIG. 7A) showed more inhibitory activity of SEB than peptide 6346 (i.e., CGKKNVTVQELDYKIRKYLVDNKKLYGC, SEQ ID NO:6) (FIG. 7B) or peptide 6348 (i.e., CMYGGVTEHEGNKKNVTVQELDYKIRKYLVDNKKLYGC, SEQ ID NO:8) (FIG. 7C).
example 3
[0151]PBMCs were isolated via Ficoll-Hypaque Solution. 150 μg of nonpolymeric 6343 peptide (i.e., CMYGGVTEGEGN, SEQ ID NO:3) and 2×105 cells in 200 μL of RPMI solution was plated in each well. The cells were incubated for one hour at 37 degrees Centigrade, with mild agitation every 15 minutes. After one hour, 2 μg of either SEB, SEC, SED, SPEC, SPEA, or TSST-1 was added to each well. The PBMCs were incubated for 72 hours and the results were measured via tritiated thymidine incorporation. The cells were collected and read on a beta counter. All experiments were run in triplicate. The results are shown in FIG. 8. Note that peptide 6313 inhibited blastogenesis of PBMCs by all of the superantigens tested.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com