Method of producing silicon oxide, negative electrode active material for lithium ion secondary battery and lithium ion secondary battery using the same
a technology of lithium ion secondary batteries and active materials, applied in silicon oxides, cell components, electrochemical generators, etc., can solve the problems of small capacity per unit mass of 372 mah/g, change in material volume, and inability to expect a further increase in capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
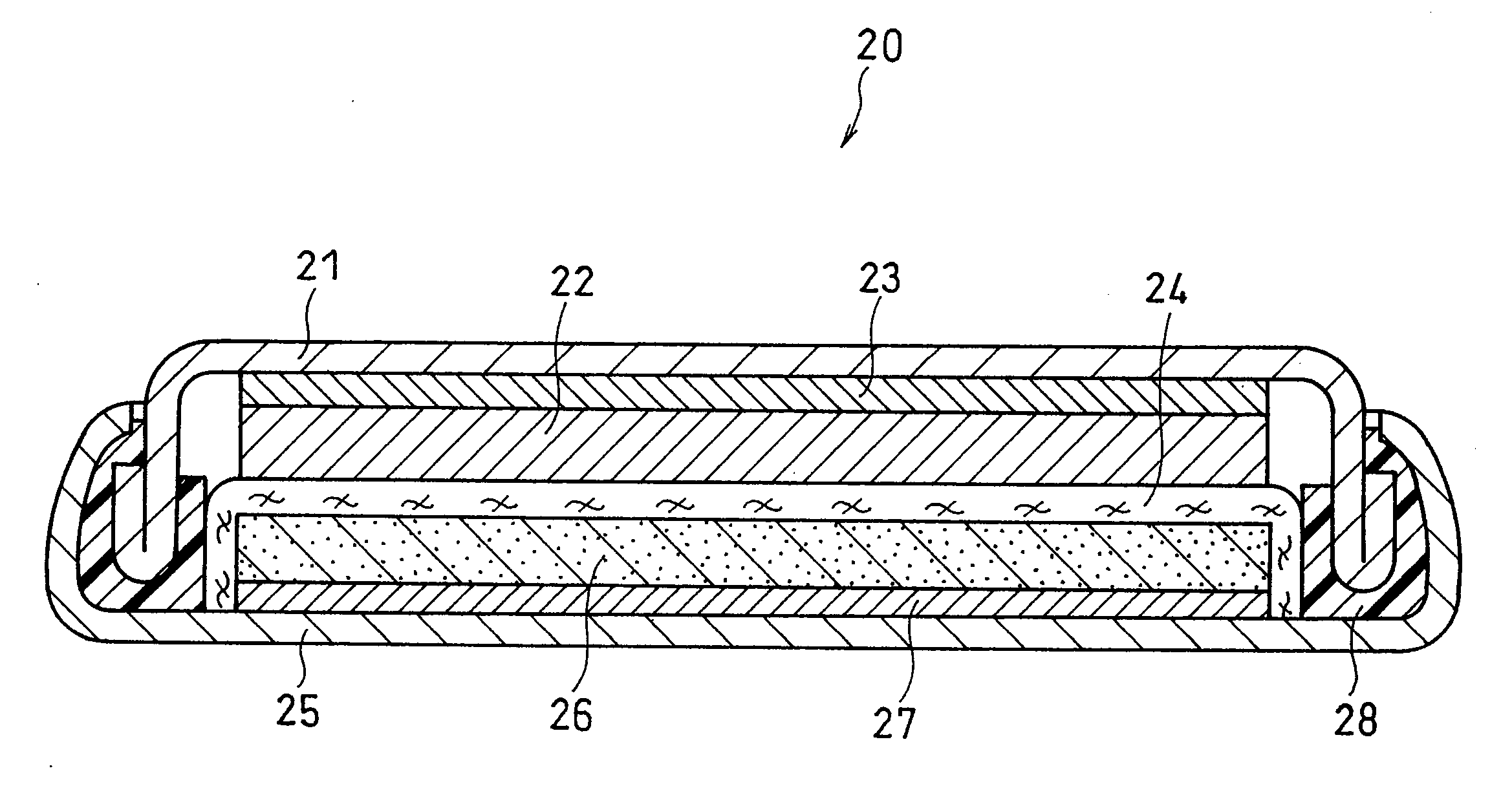
Battery 1
(Production of Positive Electrode)
[0092]With 100 parts by weight of lithium cobalt oxide (LiCoO2) having an average particle size of 5 μm was mixed, 3 parts by weight of acetylene black as a conductive material to prepare a mixture. The mixture thereby obtained and an N-methyl-2-pyrrolidone (NMP) solution in which polyvinylidene fluoride (PVdF) provided as a binder was dissolved were kneaded to obtain a paste containing a positive electrode material mixture. The NMP solution in which PVdF was dissolved was added so that 4 parts by weight of PVdF was contained in the obtained paste.
[0093]This paste was applied to one surface of a positive electrode current collector made of aluminum foil (thickness: 14 μm), dried and rolled to form a positive electrode active material layer, thus obtaining a positive electrode plate sheet.
[0094]From the obtained positive electrode plate sheet, a circular positive electrode having a diameter of 1 cm was cut out.
(Production of Negative Electro...
example 2
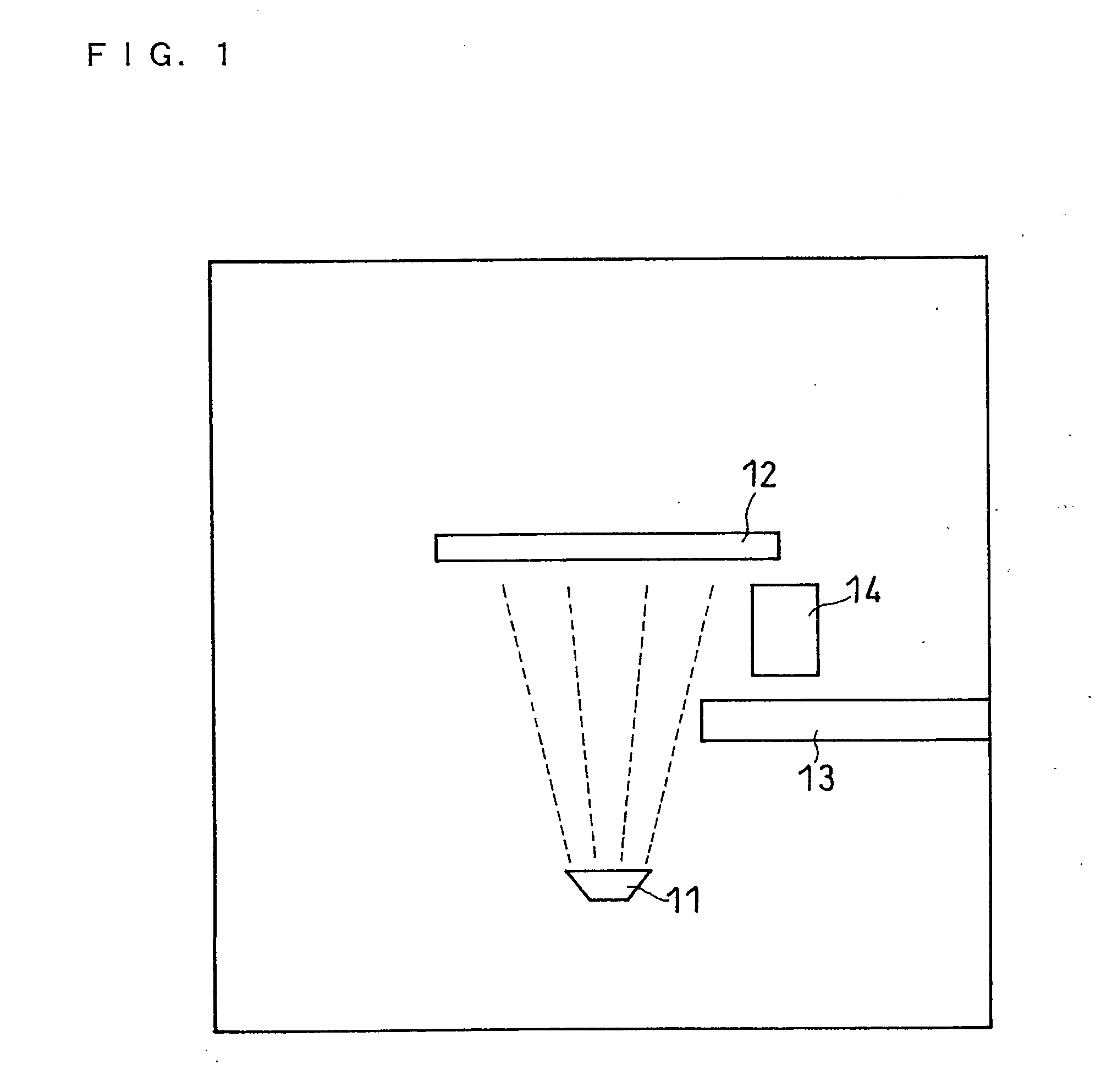
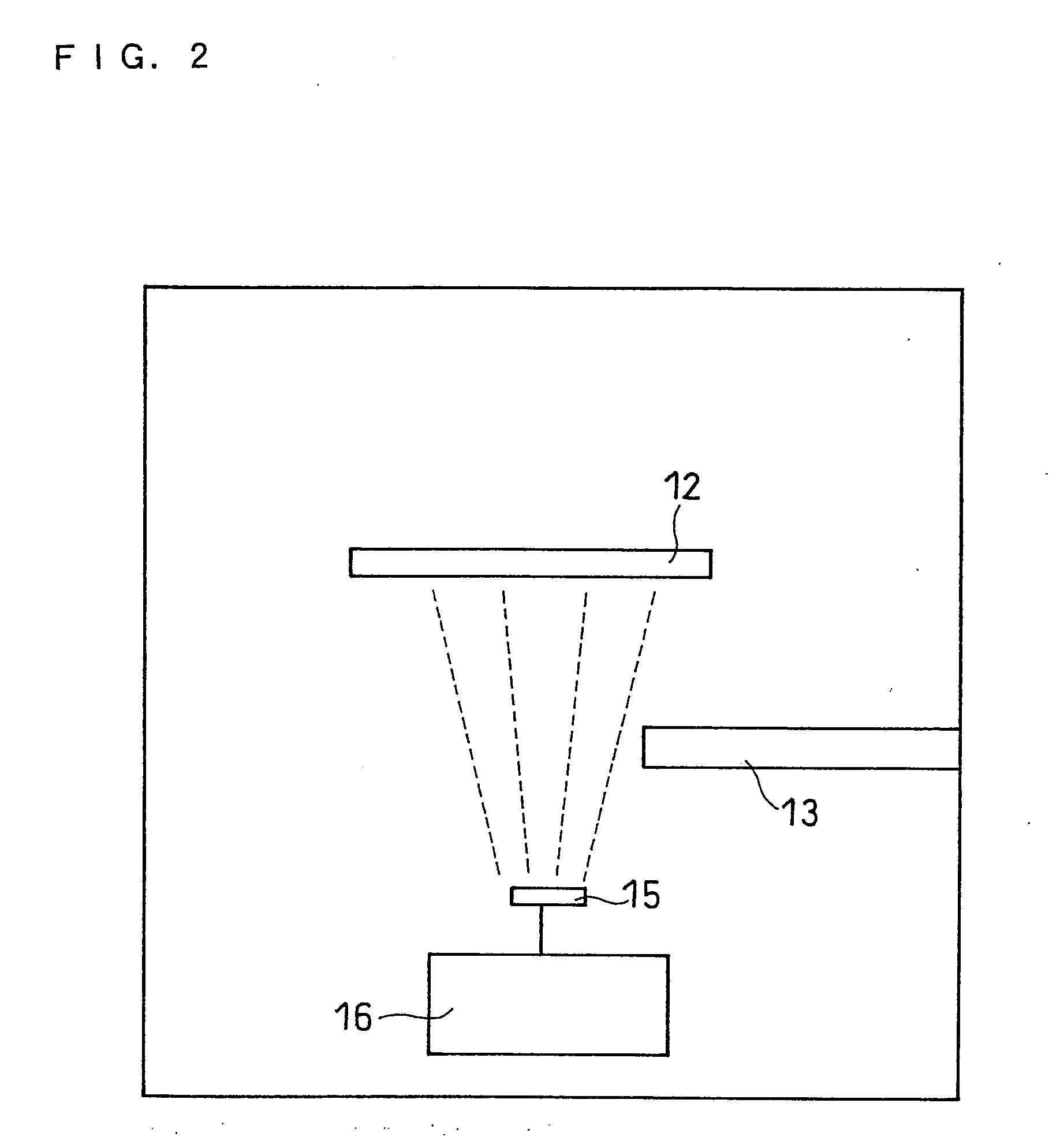
[0123]In this example, the vapor deposition apparatus shown in FIG. 1 was used and the oxygen ratio x in the negative electrode active material was changed by changing the flow rate of oxygen introduced into the vacuum chamber.
(Battery 2-1)
[0124]Battery 2-1 was made in the same manner as battery 1 except that the oxygen gas flow rate when the negative electrode active material was produced was 13 sccm, and that the thickness of the positive electrode active material layer was 1.6 times larger than that of the positive electrode active material layer in battery 1. The pressure in the vacuum chamber during the production of the negative electrode active material was 8×10−5 Torr.
[0125]The oxygen ratio in the obtained negative electrode active material was measured by the combustion method to obtain the composition of the negative electrode active material. The composition of the negative electrode active material was SiO0.1.
(Battery 2-2)
[0126]Battery 2-2 was made in the same manner as ...
example 3
Battery 3
[0134]In this example, a negative electrode active material having carbon nanofibers (CNFs) carried on its surface was produced by a method described below.
[0135]1 g of iron nitrate enneahydrate (guaranteed, available from Kanto Chemical Co., Inc.) was dissolved in 100 g of ion-exchange water. The obtained solution was mixed with the negative electrode active material used in battery 1. The mixture was agitated for 1 hour. The water content was thereafter removed from the mixture by an evaporator to provide iron nitrate containing Fe serving as a catalyst element on the surface of the negative electrode active material. The amount of iron nitrate carried thereon was 0.5 parts by weight per 100 parts by weight of the active material.
[0136]The negative electrode active material on which iron nitrate was carried was put in a ceramic reaction container and heated to 500° C. in the presence of helium gas. Thereafter, the helium gas in the reaction container was replaced with a m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap