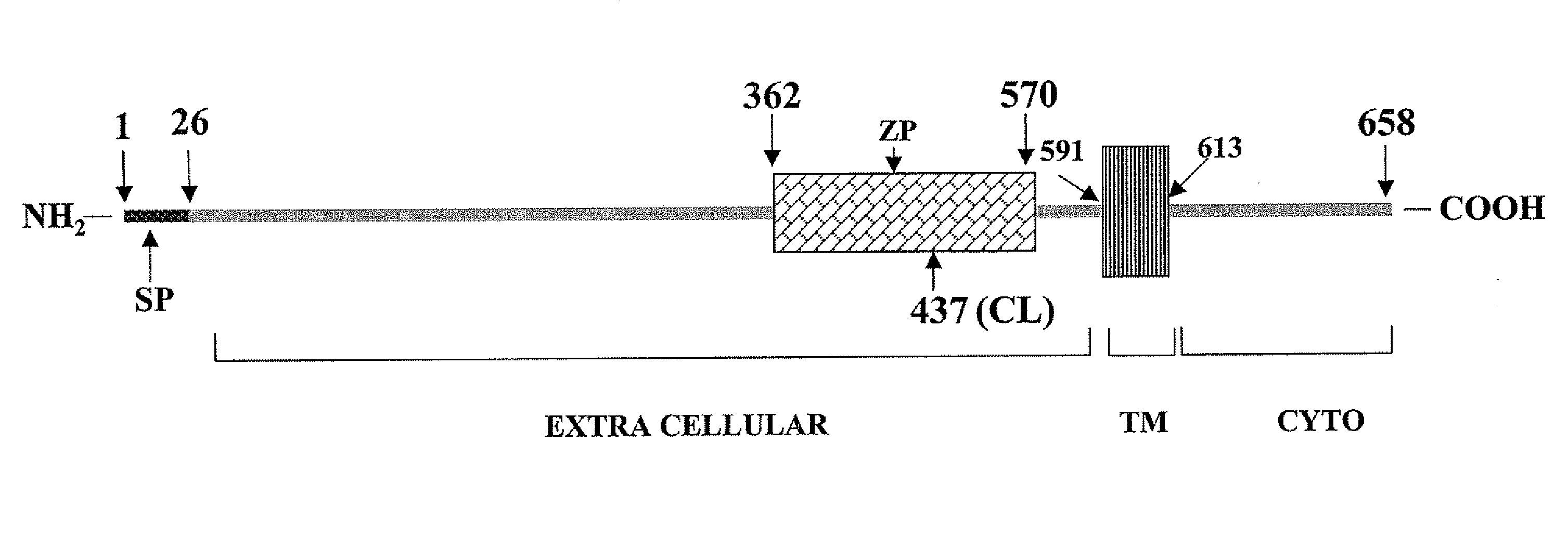
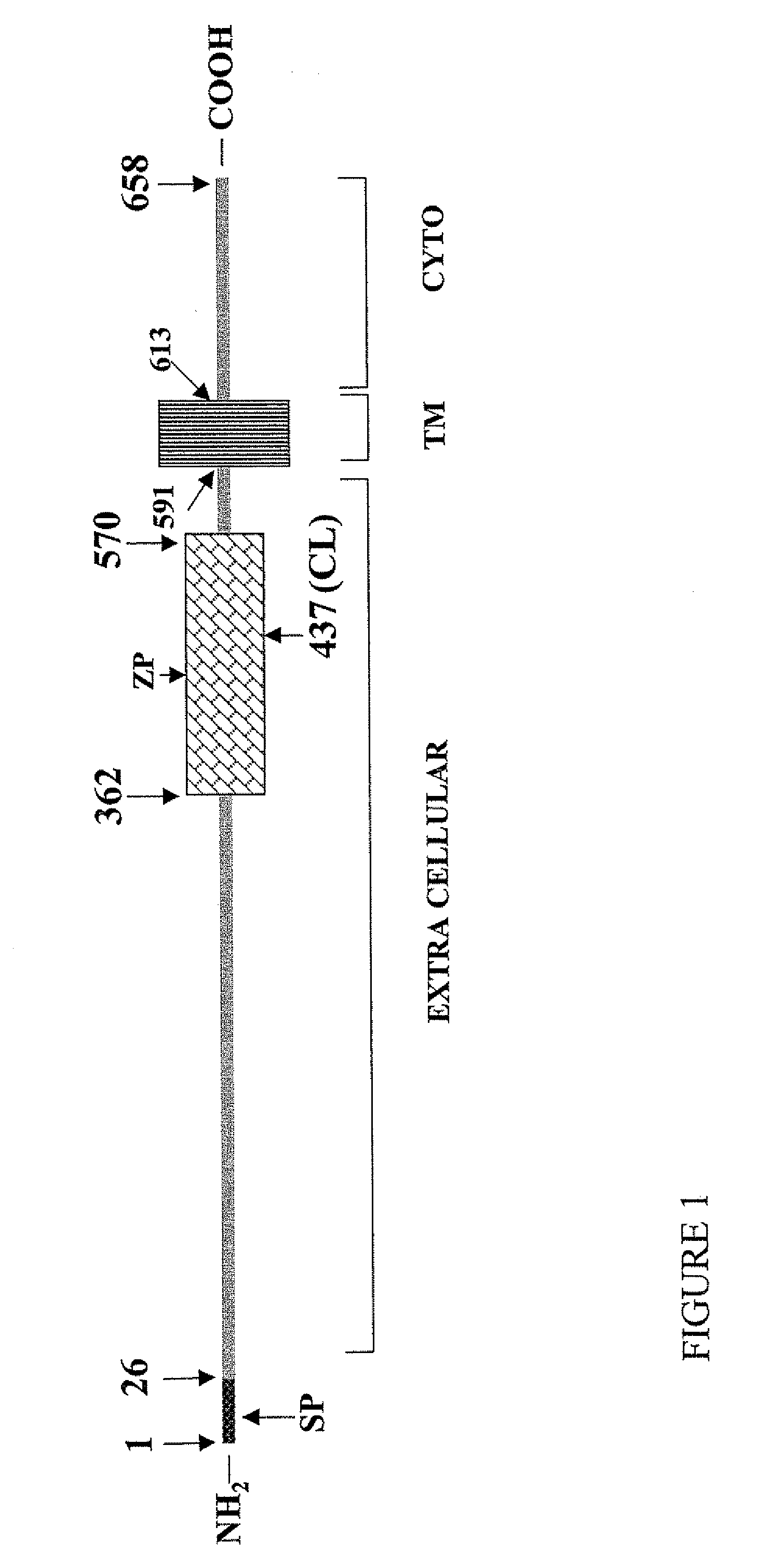
Methods of diagnosing and treating complications of pregnancy
a technology of hypertension and complications, applied in the field of pregnancy related hypertension disorders, can solve the problems of ineffective monitoring methods, no known cure for pre-eclampsia, and substantial maternal and fetal morbidity and mortality, and achieve the effects of high affinity, and reducing the elevated levels of soluble endoglin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Increased Levels of Endoglin mRNA and Protein in Pregnant Women with Pre-Eclampsia
[0248]In an attempt to identify novel secreted factors playing a pathologic role in pre-eclampsia, we performed gene expression profiling of placental tissue from 17 pregnant women with pre-eclampsia and 13 normal pregnant women using Affymetrix U95A microarray chips. We found that the gene for endoglin was upregulated in women with pre-eclampsia.
[0249]In order to confirm the upregulation of endoglin in pre-eclampsia, we performed Northern blots to analyze the placental endoglin mRNA levels (FIG. 3) and western blot analysis to measure serum protein levels of endoglin (FIG. 4) in pre-eclamptic pregnant women as compared with normotensive pregnant women. Pre-eclampsia was defined as (1) a systolic blood pressure (BP) >140 mmHg and a diastolic BP >90 mmHg after 20 weeks gestation, (2) new onset proteinuria (1+ by dipstik on urinalysis, >300 mg of protein in a 24 hour urine collection, or random urine pro...
example 2
Demonstration of a Soluble Endoglin Polypeptide in the Placentas and Serum of Pre-Eclamptic Patients
[0251]The western blot analysis used to measure the levels of endoglin protein in placentas and serum from pre-eclamptic women suggested the presence of a smaller protein (63 kDa), that was present in the placenta and serum of pre-eclamptic pregnant women. We have demonstrated that this smaller fragment is the extracellular domain of endoglin. This truncated version is likely to be shed from the placental syncitiotrophoblasts and endothelial cells and circulated in excess quantities in patients with pre-eclampsia. This soluble form of endoglin may be acting as an anti-angiogenic agent by binding to circulating ligands that are necessary for normal vascular health.
example 3
Circulating Concentrations of Soluble Endoglin in Women with Normal Versus Pre-Eclamptic Pregnancies
[0252]In order to compare the levels of circulating, soluble endoglin from the serum of normal, mildly pre-eclamptic, or severely pre-eclamptic women, we performed ELISA analysis on blood samples taken from these women. Patients were divided into mild and severe pre-eclampsia based on the presence or absence of nephritic range proteinuria (>3 g of protein on a 24 hour urine collection or urine protein / creatinine ratio greater than 3.0). The mean urine protein / creatinine ratios in the mild pre-eclampsia group were 0.94+ / −0.2 and in the severe pre-eclampsia group were 7.8+ / −2.1 (FIG. 5). ELISA was performed using a commercially available ELISA kit from R & D Systems, MN (Cat # DNDG00) as previously described (Maynard et al, J. Clin. Invest. 111:649-658, 2003).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com