Internalizing Anti-CD74 Antibodies and Methods of Use
a technology of human anticd74 and anti-cd74, which is applied in the field of humanized, chimeric and human anticd74 antibodies or fragments thereof or antibody fusion proteins, can solve the problems of limited clinical use of mll1, just as with most other promising murine antibodies, and the limitation of full-length anti-cd74 mab to limit the diagnostic and therapeutic usefulness of such antibodies and antibody conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Molecular Cloning and Sequence Elucidation for LL1 Heavy and Light Chain Variable Regions
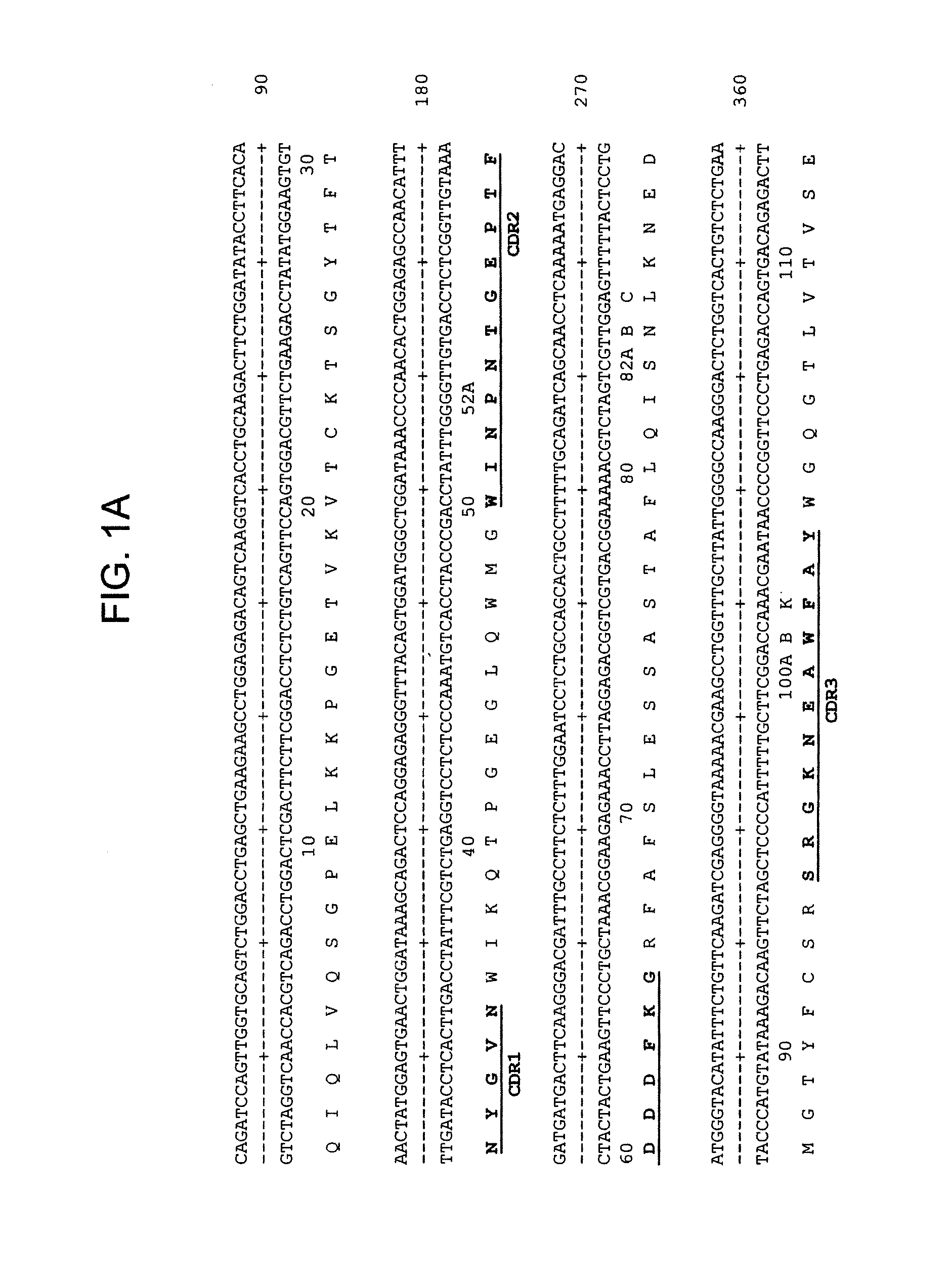
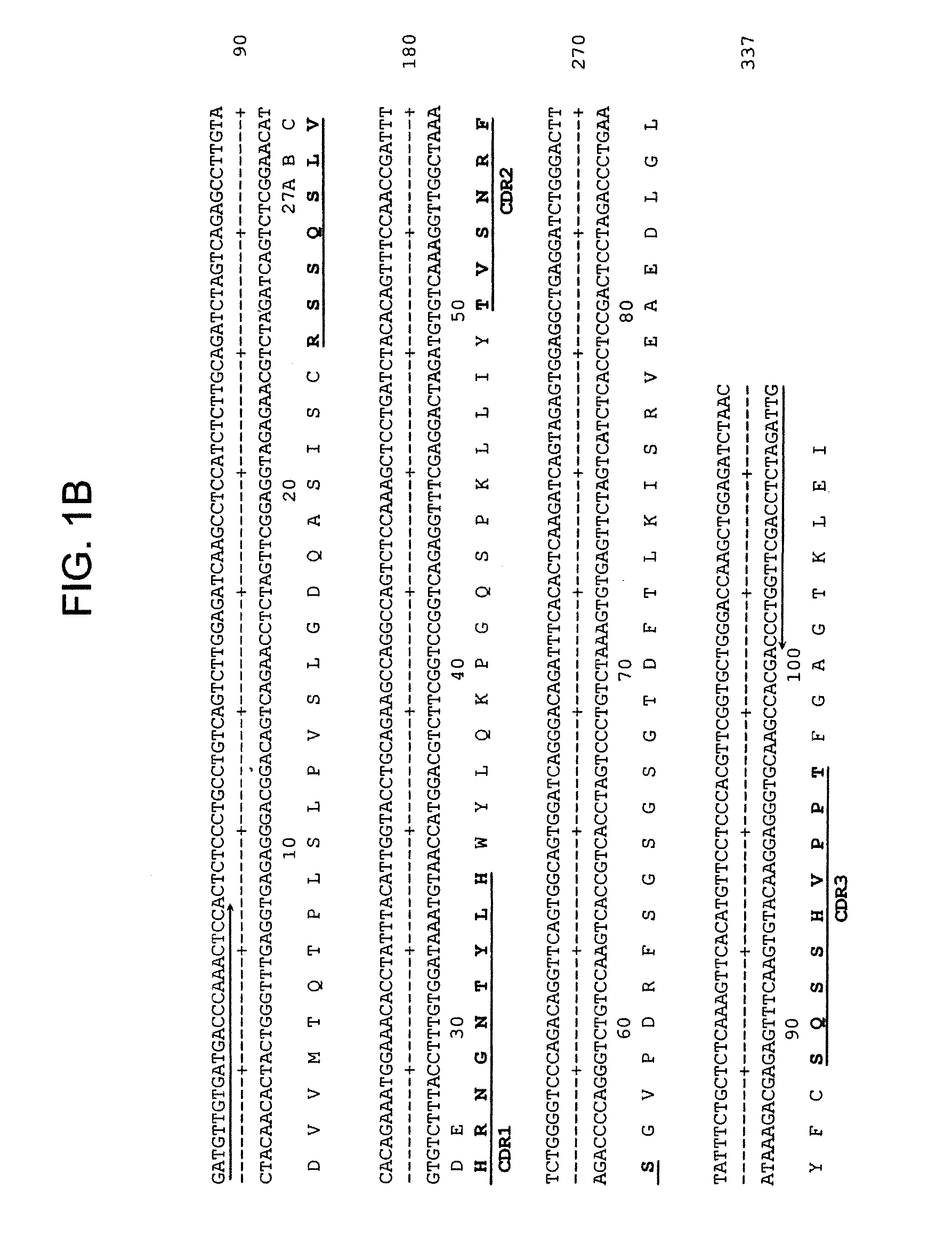
[0163]The V gene of mLL1 was obtained by RT-PCR using VK5′-4 and VK1FOR primers as described by Leung et al. 1993 and Orlandi et al. (PNAS 86:3833-3837. (1989), respectively, and cloned into pCR2.1 AT-cloning vector (Invitrogen). Multiple clones were sequenced to eliminate possible errors resulted from PCR reaction. Majority of clones (6) contained an identical murine V sequence, which was designated as LL1V and the sequence is shown in FIG. 1B. Comparison with other mouse Vk sequences revealed LL1Vk is a member of the kappa light chain subclass II.
[0164]Since RT-PCR failed to yield a full-length sequence encoding a mouse VH gene, the second cloning approach, rapid amplification of cDNA 5′-ends (5′-RACE) was employed. The adaptor-ligated cDNA prepared from LL1 hybridoma cells was amplified by PCR using a universal anchor primer (Life Technologies) and a gene specific primer, CH-1B (Leung et al. ...
example 2
Construction of the Expression Vector for Chimeric LL1
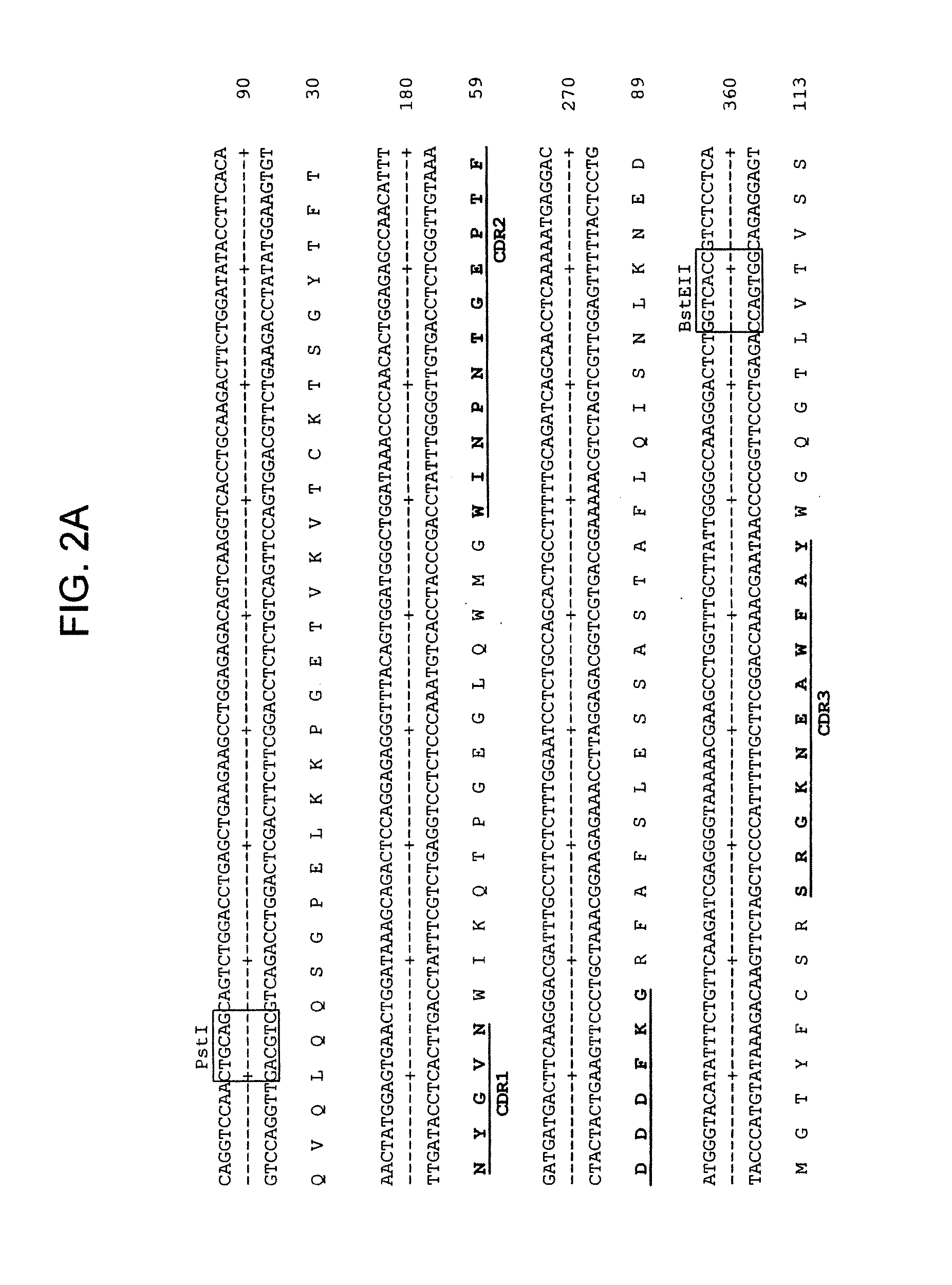
[0165]To evaluated the authenticity of the cloned Fv for LL1, a chimeric LL1 (cLL1) was constructed and expressed. The nucleotide residues 7-12 of LL1Vk were modified to a PvuII restriction site, CAGCTG, by PCR with primers LL1VK-PvuII and VK1 FOR. The resulting PCR product was digested with PvuII and BglII (partially, due to the presence of an internal BglII site in the Vk) and force-cloned into a pBR327-based staging vector (digested with PvuII and BclI) VKpBR2, which contained same Ig promoter, signal peptide sequence and convenient restriction sites to facilitate in-frame ligation of the VK PCR product as used by Orlandi et al., 1989 and Leung et al., 1994.
LL1VK-PvuII(SEQ ID NO: 23)5′GAT GTT CAG CTG ACC CAA ACT CCA CTC TCC-3′
[0166]Similarly, the nucleotide sequences at positions 10-15 and 345-351 of LL1VH were converted to PstI and BstEII, respectively, by PCR with primers LL1 B-1 and LL1F-1. The VH PCR product was then diges...
example 3
Transfection and Expression of cLL1
[0169]Approximately 30 μg of cLL1pdHL2 was linerized by digestion with SaIl and transfected into Sp2 / 0-Ag14 cells by electroporation. The transfected cells were plated into 96-well plate for 2 days and then selected for MTX resistance. Supernatants from colonies surviving selection were monitored for chimeric antibody secretion by ELISA assay. Positive cell clones were expanded and cLL1 was purified from cell culture supernatant by affinity chromatograpgy on a Protein A column.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com