Biomarkers for hypertensive disorders of pregnancy
a hypertensive disorder and biomarker technology, applied in the field of biomarkers, can solve the problems of increased perinatal mortality, increased morbidity and mortality, and severe pe, and achieve the effect of monitoring the hdp or the risk of developing the hdp
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
MASSTERMIND Discovery Platform for Discovery of New Biomarkers for PE
[0396]MASSTERMIND Experimental Setup
[0397]For biomarker discovery, we analysed the changes in protein expression using mass spectrometric detection of protein levels using our previously published COFRADIC™ technology platform (substantially as described inter alia in WO 02 / 077016 and in Gevaert et al. 2003, Nat Biotechnol 21(5): 566-9).
[0398]All plasma samples were depleted for the most abundant proteins using commercially available affinity-based chromatographic columns (e.g. Agilent Technologies). Depletion efficiency of albumin and immunoglobulin G (IgG) was checked using Western Blot analysis. Samples were prepared for MASStermind analysis according to the standard N-terminal COFRADIC procedures. Samples and controls were differentially labelled by trypsin mediated incorporation of 18O / 16O at the C-terminus of every tryptic peptide. After N-terminal peptide sorting, NanoLC separations followed by direct spotti...
example 2
Identification of Markers Useful in PE
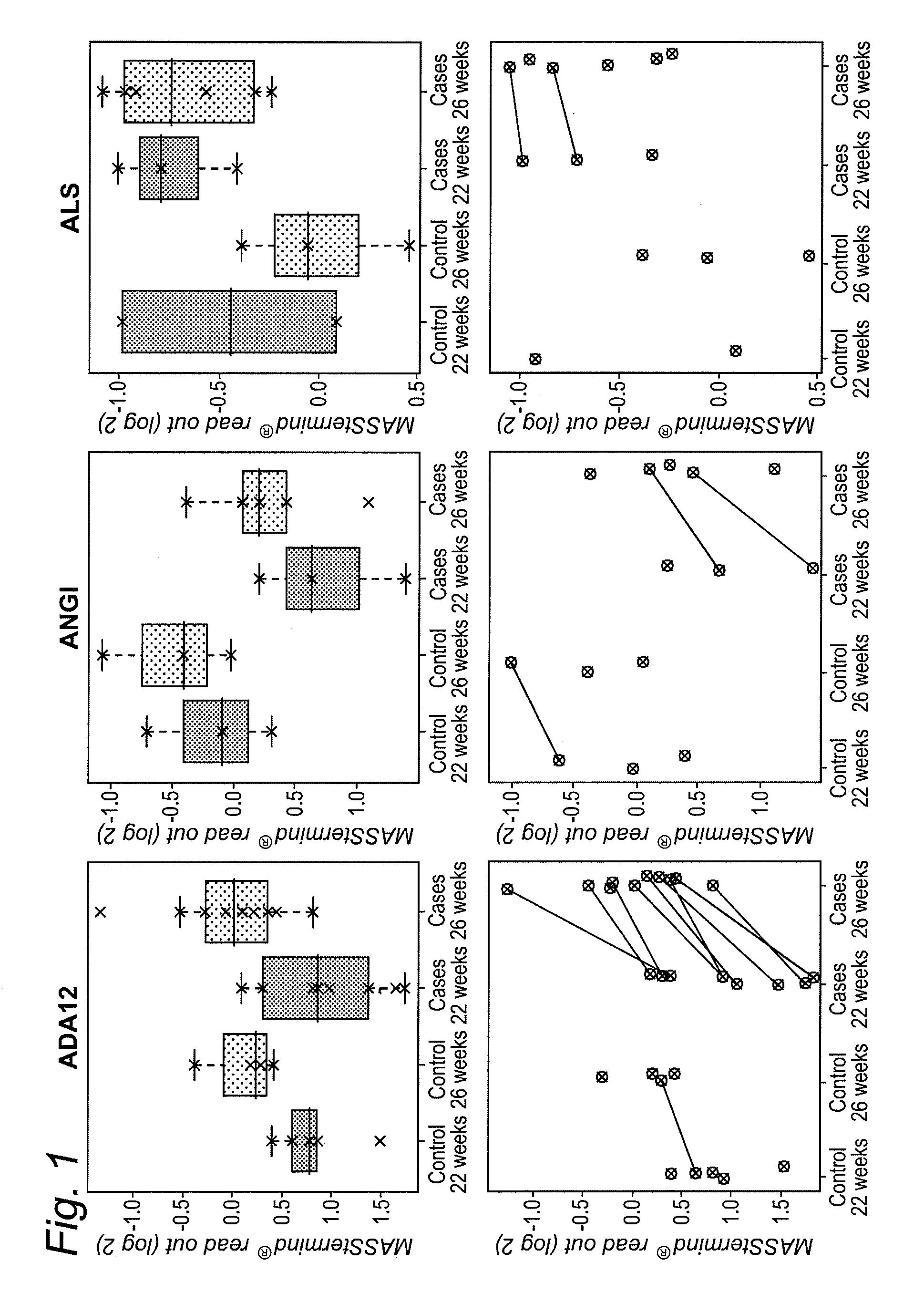
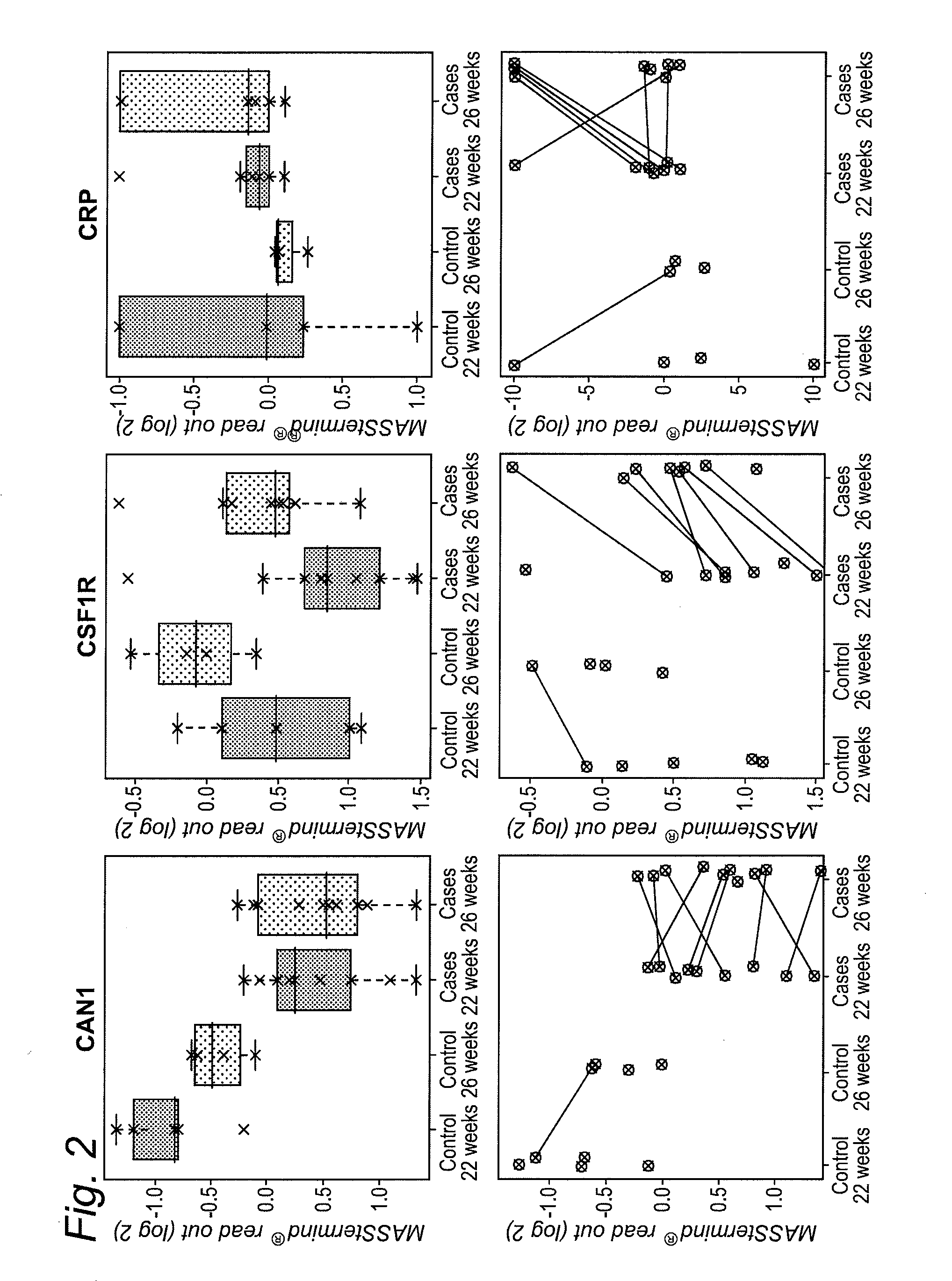
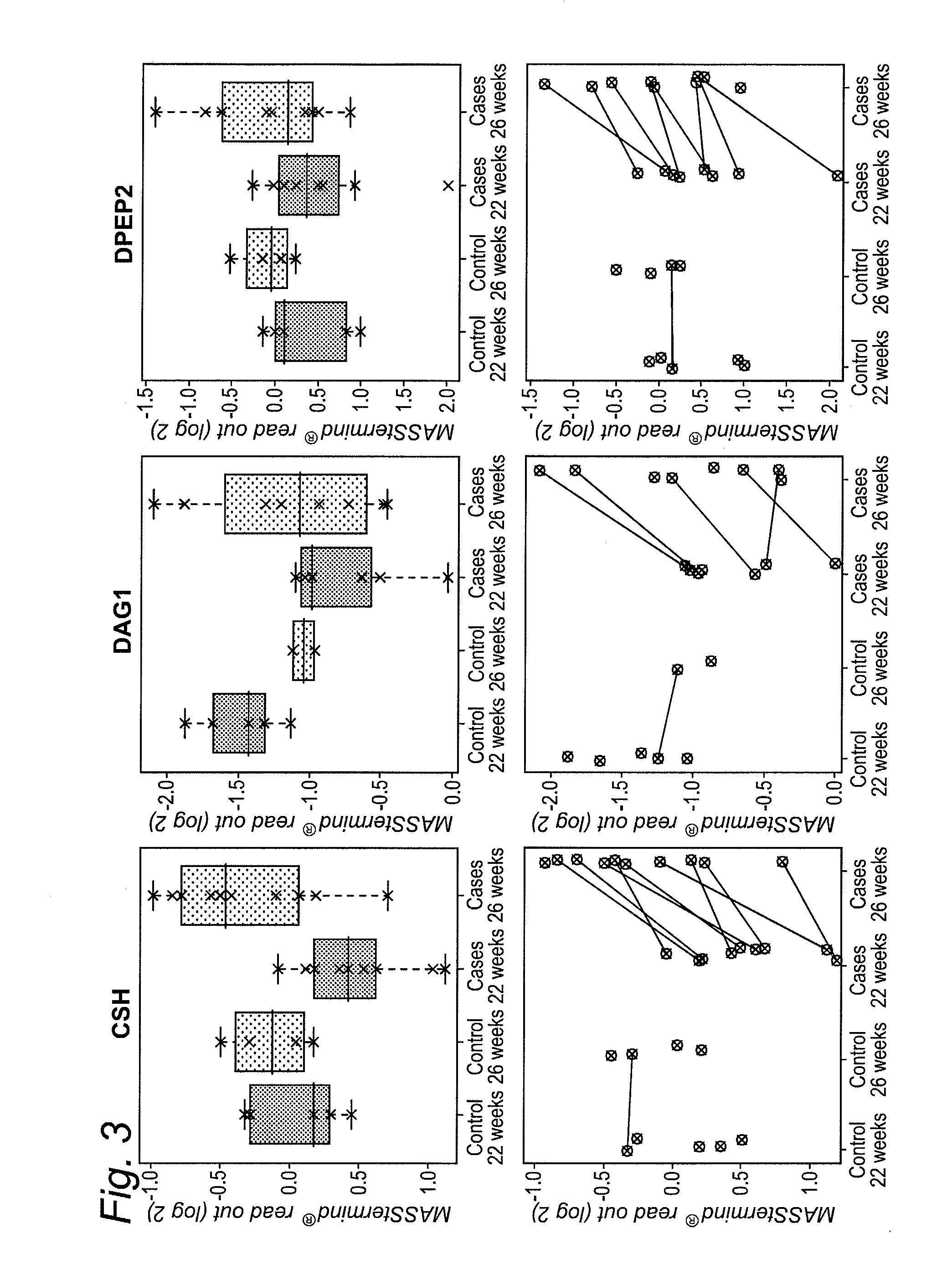
[0401]The study design aimed to identify protein-based biomarkers allowing to discern pregnant women destined to develop preeclampsia (PE) later in their pregnancy (cases) from women that will not develop PE later in their pregnancy (controls).
[0402]Plasma samples were obtained from pregnant women at two distinct time points within their pregnancy (22 and 26 weeks of gestation), i.e., 2 samples per individual were obtained (FIG. 17). At the time of sampling any clinical sign of later PE are still absent within the cases.
[0403]The MASStermind discovery study applied was a so-called “Reference design” wherein all samples were compared to a common reference, which constitutes a mixture of most samples used in the study.
[0404]Four groups of samples were defined: group 1 (PE-destined women at 22 weeks of gestation, n=10), group 2 (PE-destined women at 26 weeks of gestation, n=10), group 3 (control pregnant women at 22 weeks of gestation, n=5), and gr...
example 3
MASSTERCLASS Targeted Protein Quantification
[0409]The following describes one exemplary way of targeted protein quantification in samples.
[0410]MASSTERCLA SS Experimental Setup
[0411]MASSterclass assays use targeted tandem mass spectrometry with stable isotope dilution as an end-stage peptide quantitation system (also called Multiple Reaction Monitoring (MRM) and Single Reaction Monitoring (SRM)). The targeted peptide is specific (i.e., proteotypic) for the specific protein of interest. i.e., the amount of peptide measured is directly related to the amount of protein in the original sample (see, e.g., the peptides in Table 1). To reach the specificity and sensitivity needed for biomarker quantitation in complex samples, peptide fractionations precede the end-stage quantitation step.
[0412]A suitable MASSTERCLASS assay may include the following steps:[0413]Plasma / serum sample[0414]Depletion of human albumin and IgG (complexity reduction on protein level) using affinity capture with ant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| blood pressure | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com