Interleukin-13 receptor alpha 2 peptide-based brain cancer vaccines
a technology of interleukin-13 receptor and brain cancer, which is applied in the direction of antibody medical ingredients, peptide/protein ingredients, immunological disorders, etc., can solve the problems of lack of effective treatment options, difficult treatment of brain tumors, and more difficult treatment of malignant gliomas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
7.1 Example 1
[0227]This example demonstrates the identification of modified peptides for IL-13Rα2345-353 that enhance induction of the CTL response against native IL-13Rα2345-353.
[0228]Three modified peptides were synthesized as listed in Table 1. The binding capability of these modified peptides was assessed using an HLA-A2 transfected T2 cell line. Aliquots of T2 cells were incubated with modified peptides or IL-13Rα2345-353 at 1 nM overnight, and then examined for the surface expression levels of HLA-A2 on T2 cells by flow cytometry. Since stable binding of HLA-A2 with peptide epitopes further stabilizes the surface expression of HLA-A2 (Francini et al., 2002; Alves et al., 2003), quantitative expression levels of HLA-A2, which is indicated by Mean Fluorescence Intensity (MFI) in Table 1, correlate with the binding affinity of the peptide-epitopes that are co-incubated with the T2 cells. The modified peptides V9 and A1V9 possess higher binging affinity to HLA-A2 than the native I...
example 2
7.2 Example 2
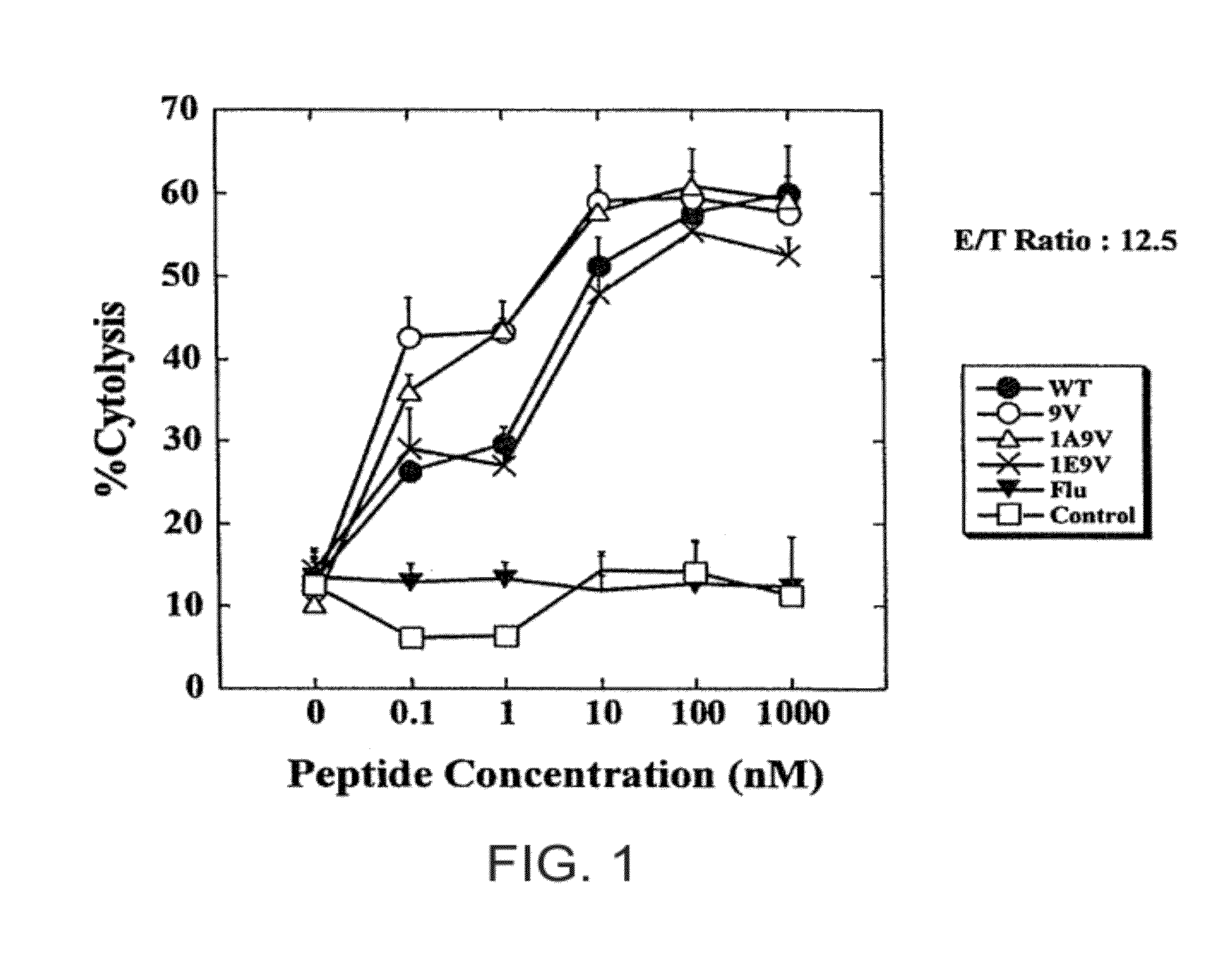
[0229]This example demonstrates that CTL induced by the agonist analogue V9 recognized peptide IL-13Rα2345-353 presented on HLA-A*0201 more efficiently than CTL induced by the wild type peptide.
[0230]Dendritic cells (DCs) derived from HLA-A*0201+ glioma patients were pulsed with either V9, A1V9, E1V9, a control influenza (flu), or the wild type peptide (10 μg / ml), and used to stimulate autologous CD8+ T cells. On day 7, the individual responder cell cultures were then restimulated once with autologous DCs loaded with the corresponding peptide used in the primary stimulation. Specific CTL activity of the induced T cell lines was first tested with T2 cells loaded with the wild type IL-13Rα2345-353, or no peptide on day 10.
[0231]As depicted in FIG. 1, the T cells that had been stimulated with either wild type (IL-13R) or agonist analogues (V9, A1V9 and E1V9) efficiently lysed T2 target cells pulsed with 100 ng / ml wild type IL-13Rα2345-353; whereas only low background lysis...
example 3
7.3 Example 3
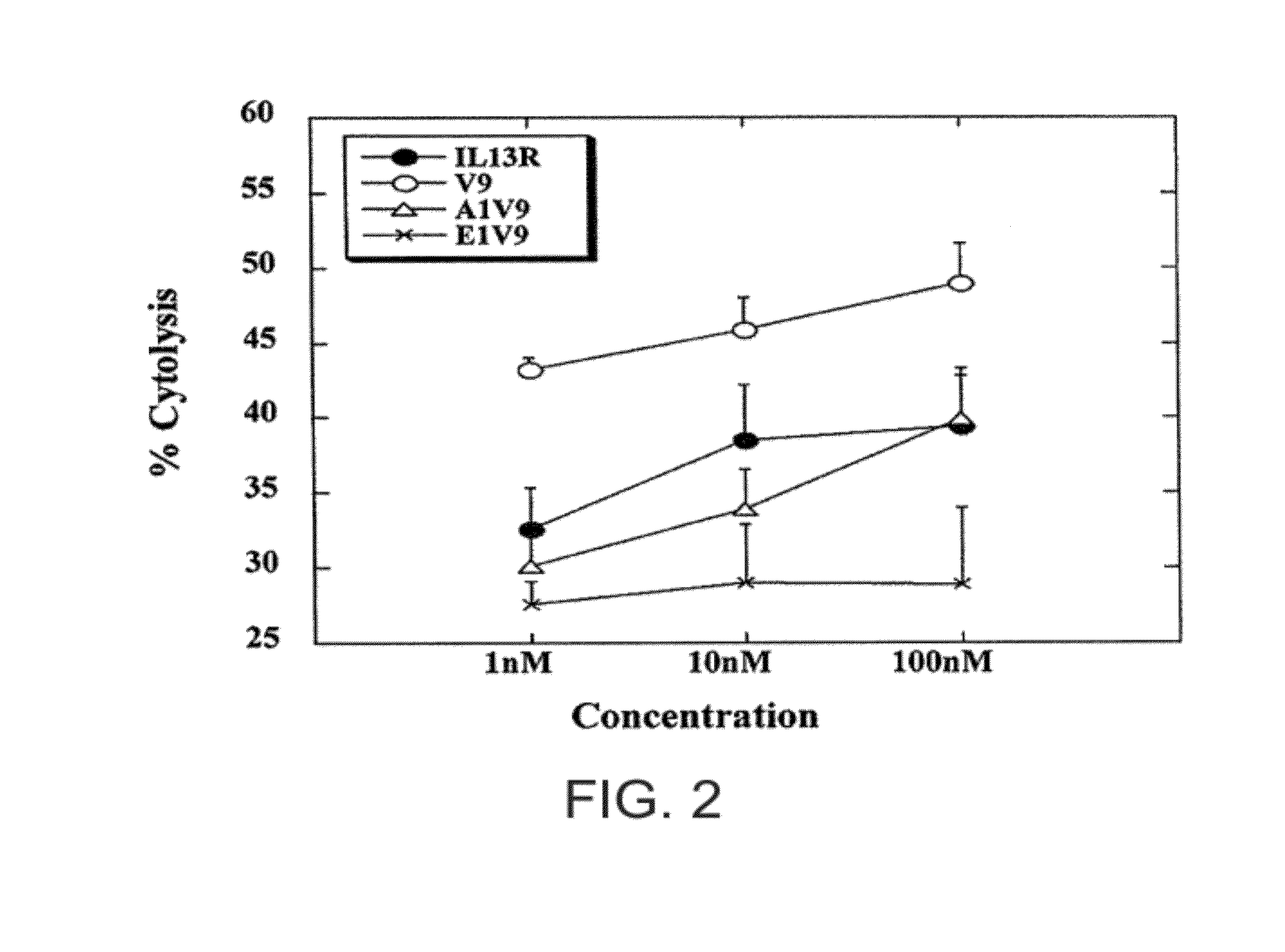
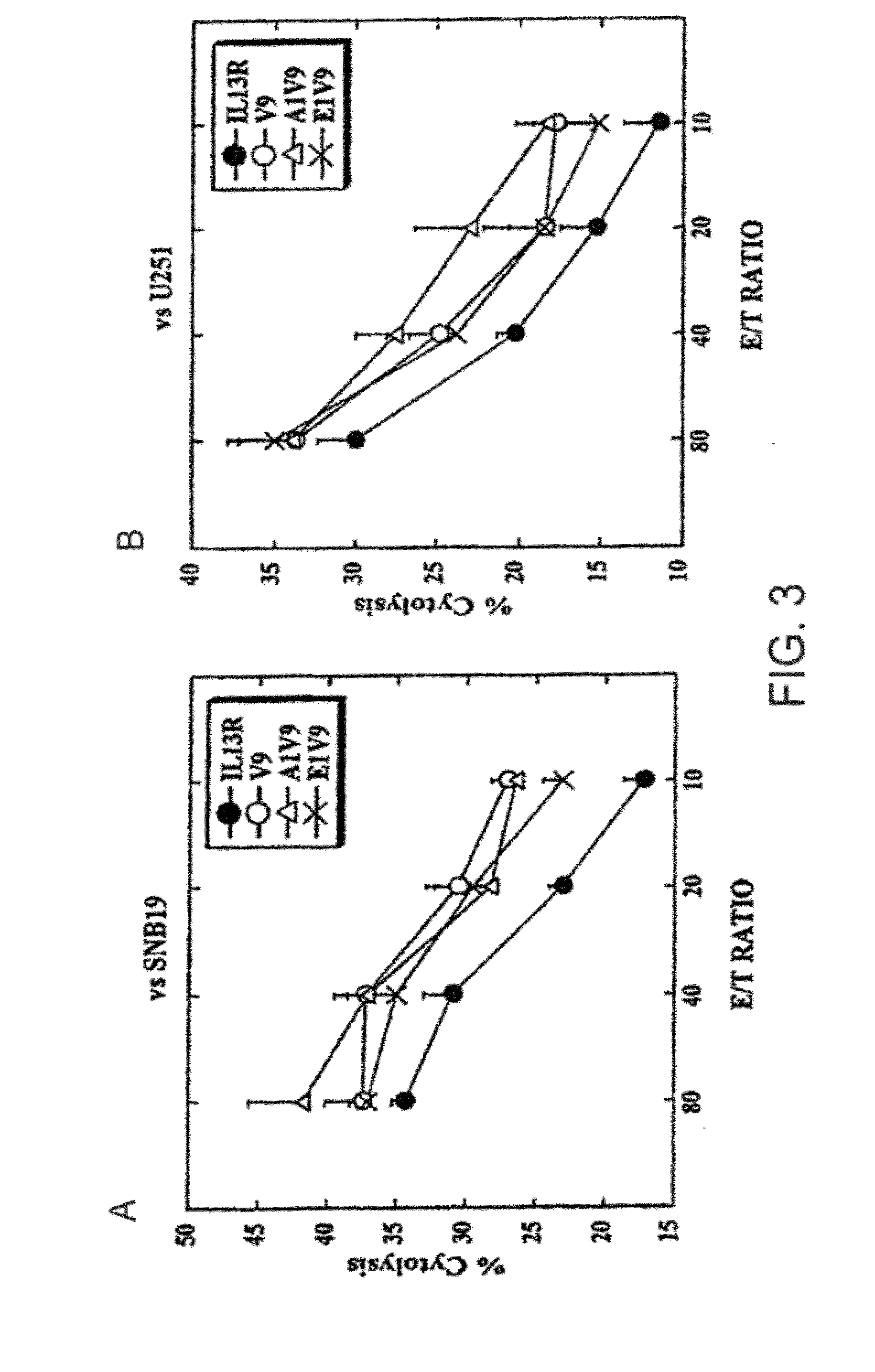
[0233]This example demonstrates that CTL induced by modified peptides lysed HLA-A2+ glioma cells that express IL-13Rα2 more efficiently than CTL induced by the native peptide.
[0234]The ability of modified peptides, such as IL-13Rα2-V9, to enhance the CTL activity against HLA-A2+ human glioma cells that endogenously expressed and presented IL-13Rα2-derived epitopes was examined. Human glioma cell lines U251 and SNB19 express HLA-A2 and IL-13Rα2, whereas human glioma cell line A172 expresses IL-13Rα2 but not HLA-A2 (Okano et al., 2002). Therefore, U251 and SNB 19 were used as relevant target glioma cells, while A172 served as a negative control line to demonstrate HLA-A2-restriction of the response.
[0235]The lytic ability of the peptide-induced CTL lines against these glioma cells was examined using 4-hr 51Cr-release assays. As illustrated in FIG. 3, the U-251 and SNB19 cell lines were highly susceptible to cytotoxic activity of all the CTL lines that had been induced wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com