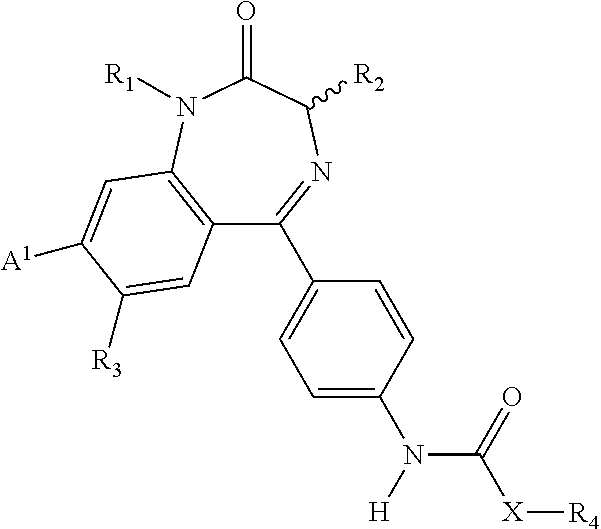
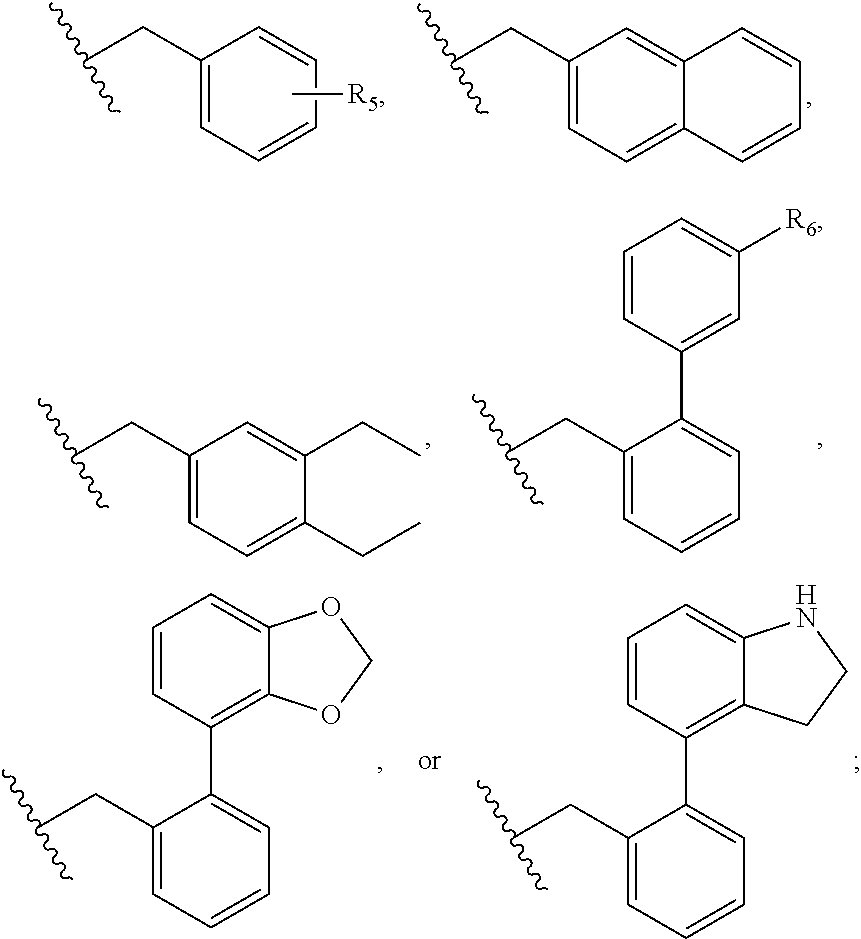
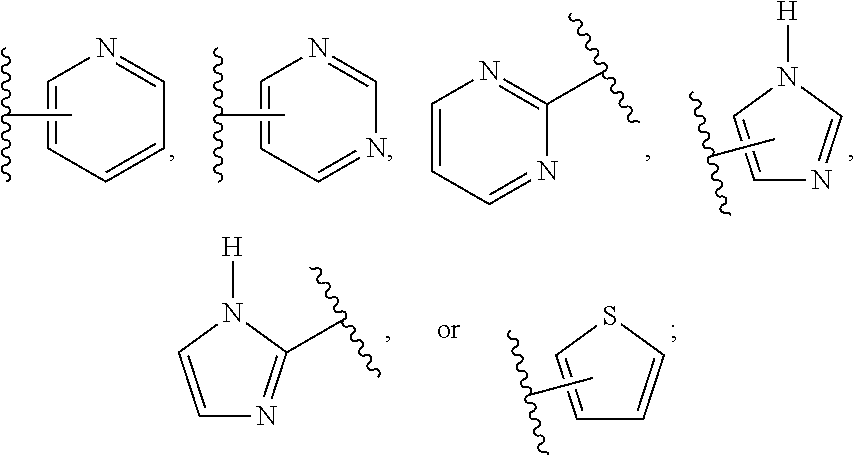
Compositions and methods relating to novel compounds and targets thereof
a technology of novel compounds and compounds, applied in the field of new chemical compounds, can solve the problems of limiting efficacy, serious drawbacks of existing cytotoxic chemotherapeutic agents, and serious deleterious effects in the organism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0274]This Example shows the synthesis of the following compound
[0275]The compound was synthesized in accordance with the following reaction scheme.
example 2
[0276]It is contemplated that certain compounds of the invention could be made using the synthetic schemes shown below. Consistent with art-recognized terminology, the abbreviation “PMB-Cl” refers to 4-methoxybenzoyl chloride.
example 3
[0277]The following compounds were assayed for their ability to cause apoptosis of Ramos B cells, based on the procedures described in Blatt et al. J. Clin. Invest. 110: 1123-1132 (2002). Each of the following compounds were found to have an EC50<5 μM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com