Method of producing antibodies
a technology of antibody and recombinant protein, which is applied in the field of producing antibodies, can solve the problems of difficult to obtain dna expression in cells, alterations and/or damage to the genomic dna of host cells, etc., and achieve the effects of increasing the efficiency of recombinantly expressed protein translation, and increasing the production of recombinantly expressed protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Modified mRNA
[0170]Modified mRNAs (modRNAs) according to the invention were made using standard laboratory methods and materials. The open reading frame (ORF) of the gene of interest is flanked by a 5′ untranslated region (UTR) containing a strong Kozak translational initiation signal and an alpha-globin 3′ UTR terminating with an oligo(dT) sequence for templated addition of a polyA tail. The modRNAs were modified with pseudouridine (ψ) and 5-methyl-cytidine (5meC) to reduce the cellular innate immune response. Kariko K et al. Immunity 23:165-75 (2005), Kariko K et al. Mol Ther 16:1833-40 (2008), Anderson B R et al. NAR (2010).
[0171]The cloning, gene synthesis and vector sequencing was performed by DNA2.0 Inc. (Menlo Park, Calif.). Vector sequences and insert sequences are set forth in SEQ ID NOs: 5-8. The ORFs were restriction digested using XbaI or HindIII and used for cDNA synthesis using tailed-PCR. This tailed-PCR cDNA product was used as the template for the modif...
example 2
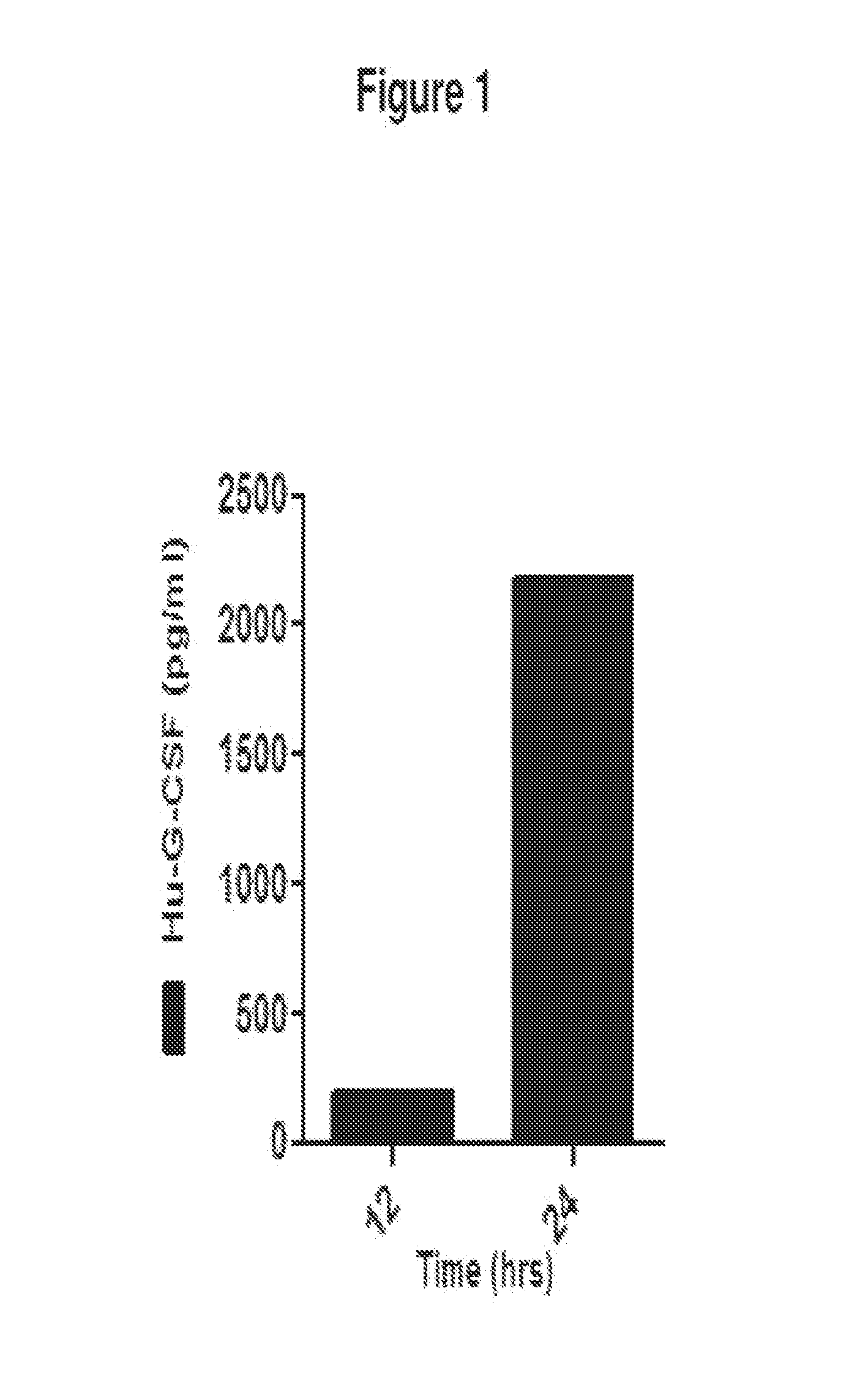
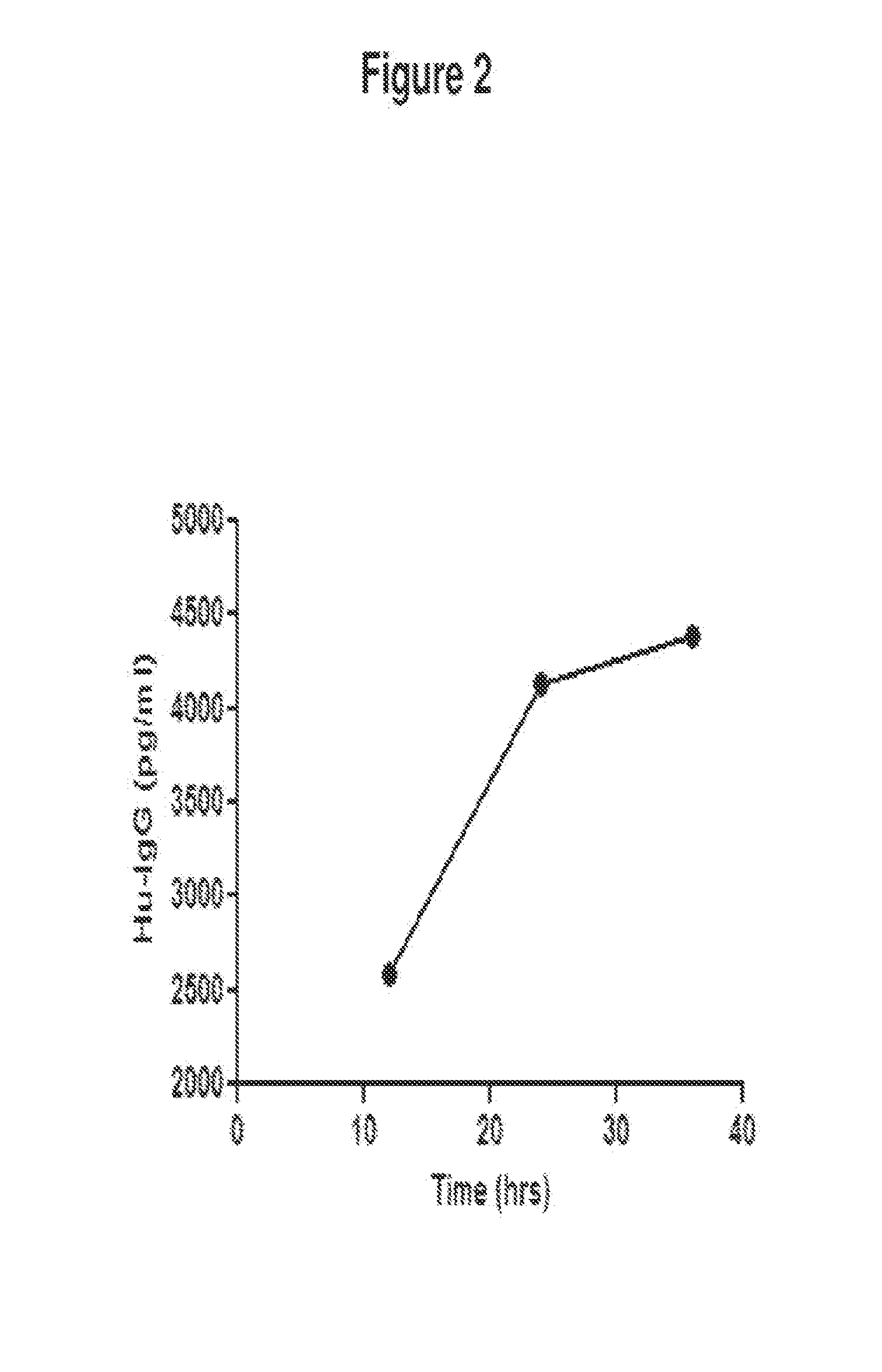
De novo Generation of a Mammalian Commercial Production Cell Line Expressing Human G-CSF as a Therapeutic Agent in Model Bioreactor
[0176]The nucleic acid sequence for the precursor of human granulocyte colony stimulating factor (G-CSF) is set forth in SEQ ID NO: 1:
(SEQ ID NO: 1)agcttttggaccctcgtacagaagctaatacgactcactatagggaaataagagagaaaagaagagtaagaagaaatataagagccaccatggccggtcccgcgacccaaagccccatgaaacttatggccctgcagttgctgctttggcactcggccctctggacagtccaagaagcgactcctctcggacctgcctcatcgttgccgcagtcattccttttgaagtgtctggagcaggtgcgaaagattcagggcgatggagccgcactccaagagaagctctgcgcgacatacaaactttgccatcccgaggagctcgtactgctcgggcacagcttggggattccctgggctcctctctcgtcctgtccgtcgcaggctttgcagttggcagggtgcctttcccagctccactccggtttgttcttgtatcagggactgctgcaagcccttgagggaatctcgccagaattgggcccgacgctggacacgttgcagctcgacgtggcggatttcgcaacaaccatctggcagcagatggaggaactggggatggcacccgcgctgcagcccacgcagggggcaatgccggcctttgcgtccgcgtttcagcgcagggcgggtggagtcctcgtagcgagccaccttcaatcatttttggaagtctcgtaccgggtgctgagacatcttgcgcagccgtgaagcgctgccttctg...
example 3
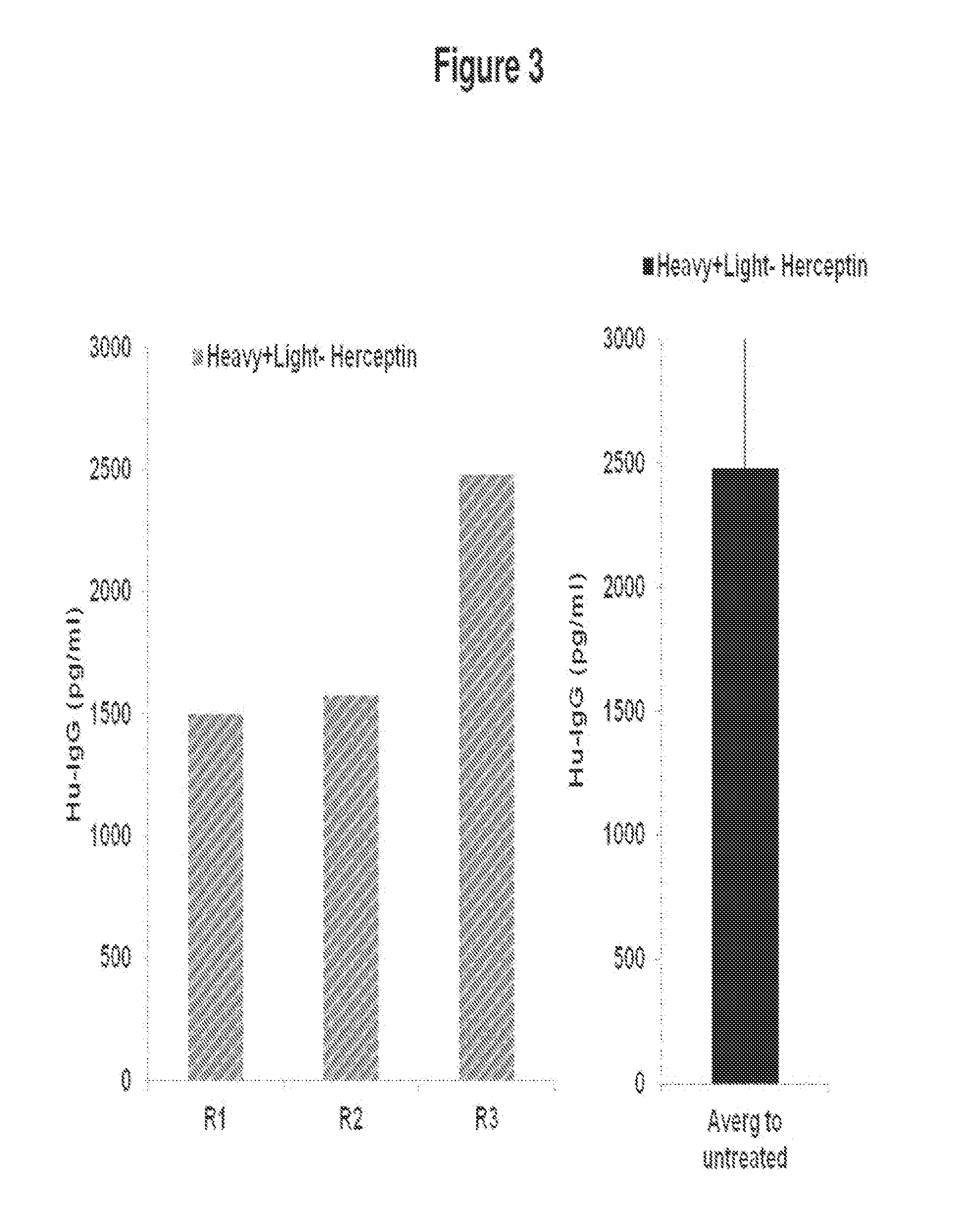
De novo Generation of a Mammalian Commercial Production Cell Line Expressing Humanized IgG Antibodies (Trastuzumab and Rituximab) as a Therapeutic Agent in Model Bioreactor
[0180]The nucleic acid sequence for the Heavy Chain of Rituximab is set forth in SEQ ID NO: 4:
(SEQ ID NO: 4)CTCGTACAGAAGCTAATACGACTCACTATAGGGAAATAAGAGAGAAAAGAAGAGTAAGAAGAAATATAAGAGCCACCATGGCCGTGATGGCGCCGAGGACCCTGGTGCTCTTGCTCACGGGTGCCTTGGCCCTCACGCAAACATGGGCGGGACAGGCGTACTTGCAGCAGTCAGGGGCAGAACTCGTAAGGCCCGGAGCGTCGGTGAAGATGTCGTGTAAAGCGTCGGGCTATACTTTCACATCGTACAACATGCACTGGGTCAAACAGACGCCCCGACAAGGGCTGGAGTGGATTGGAGCTATCTACCCCGGTAACGGGGATACGTCGTACAACCAGAAGTTTAAGGGGAAGGCGACTCTTACTGTCGACAAGTCGTCCTCCACCGCCTATATGCAGCTGTCGAGCCTGACTTCGGAAGATTCAGCGGTGTACTTTTGTGCGCGCGTGGTCTATTACTCAAATTCGTATTGGTATTTCGATGTGTGGGGTACGGGGACCACTGTGACCGTGTCAGGACCCTCGGTATTCCCCCTCGCGCCTAGCTCAAAGTCCACCTCCGGGGGAACAGCCGCCTTGGGTTGCTTGGTAAAGGACTATTTCCCCGAGCCCGTCACAGTGAGCTGGAACTCCGGGGCACTGACATCGGGAGTGCACACGTTTCCCGCGGTACTTCAGTCATCAGGACTCTACTCGCTGTCAAGCGTGGTCACGGTGC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com