Implants and Methods for Correcting Tissue Defects
a tissue defect and implant technology, applied in the field ofmosaic implants, can solve the problems of high risk of scaffold failure, difficulty in healing of bone defects, and problems with unhealed defects, and achieve the effects of improving the combined bone in-growth and mechanical properties, high mechanical strength, and high mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example 1
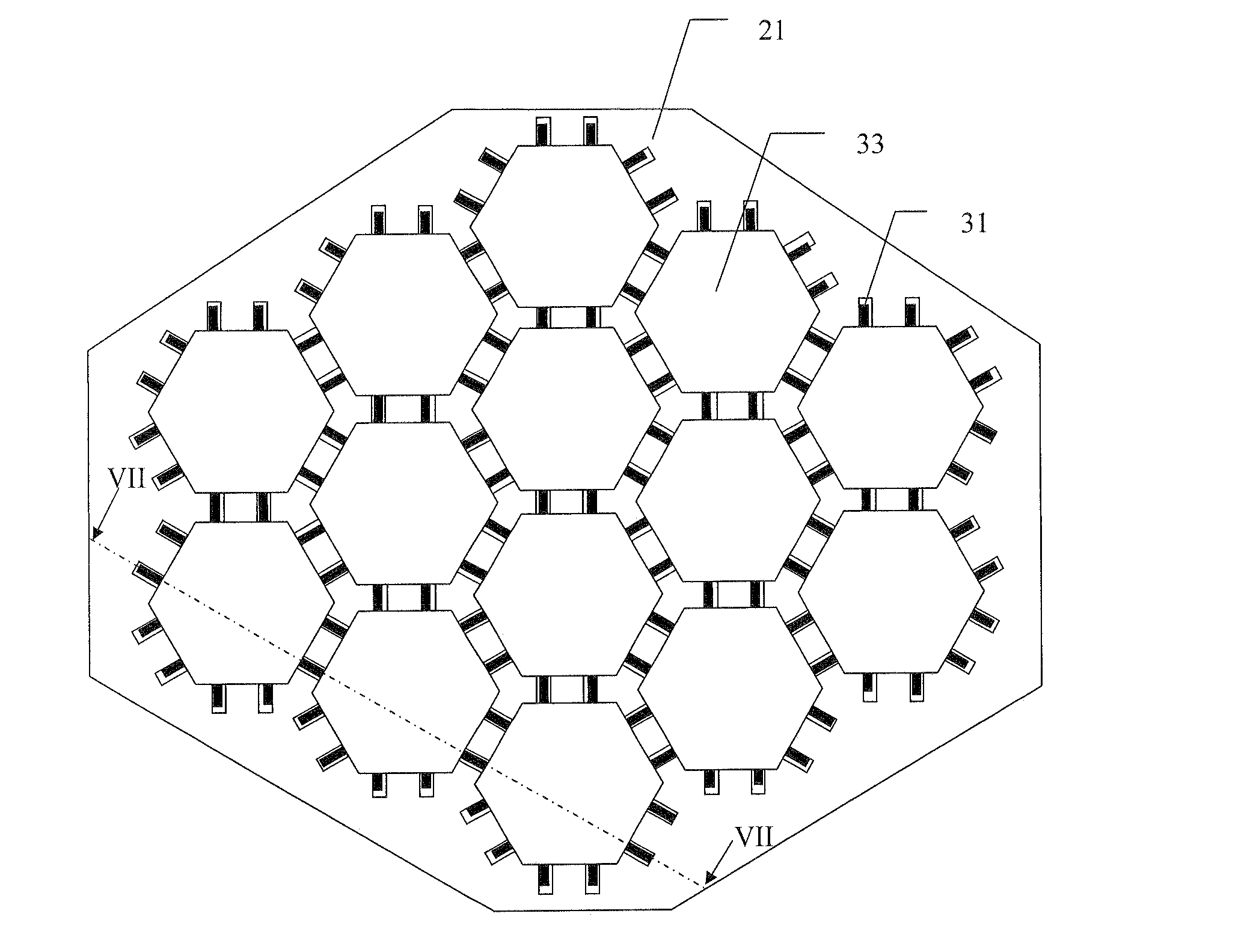
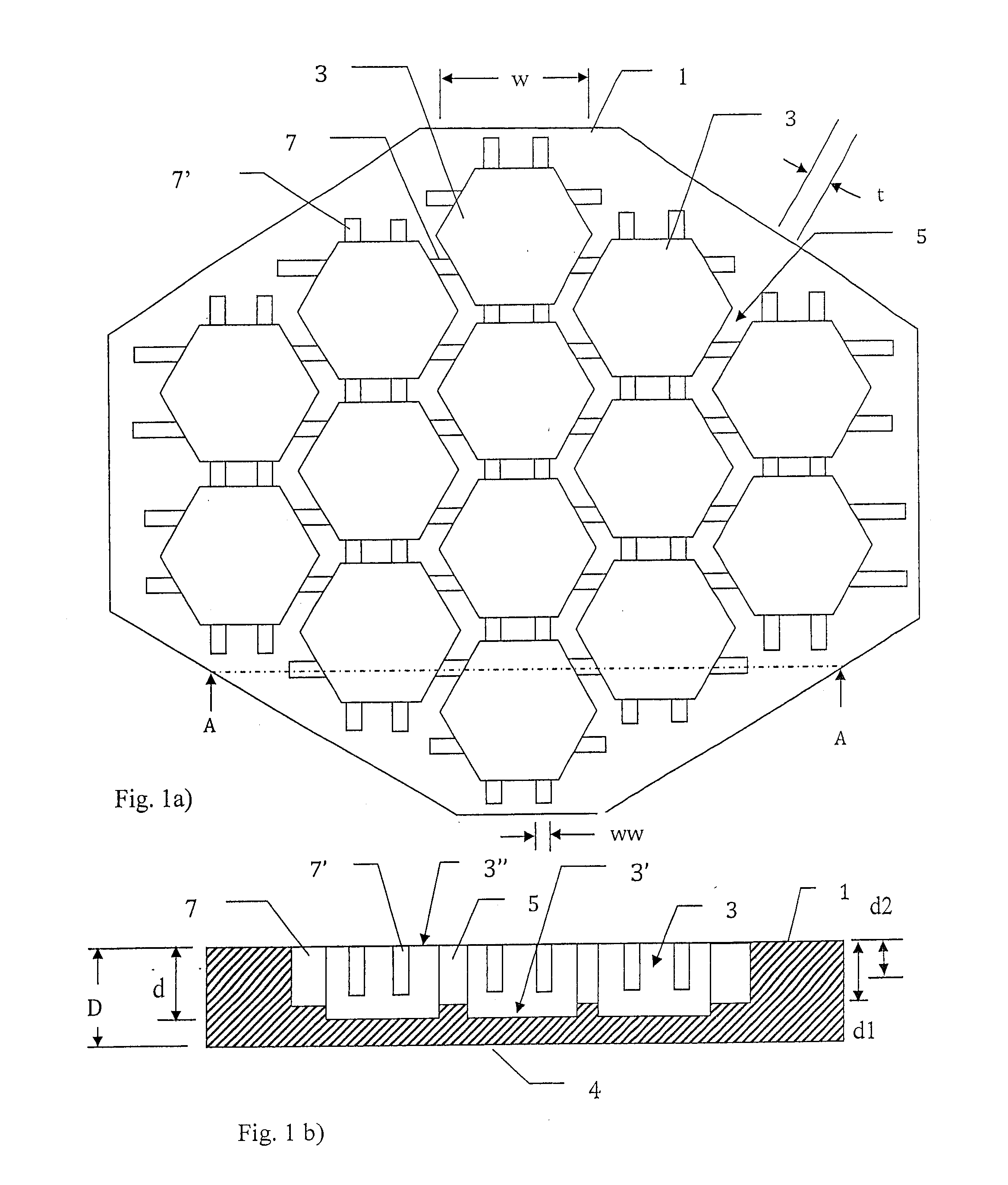
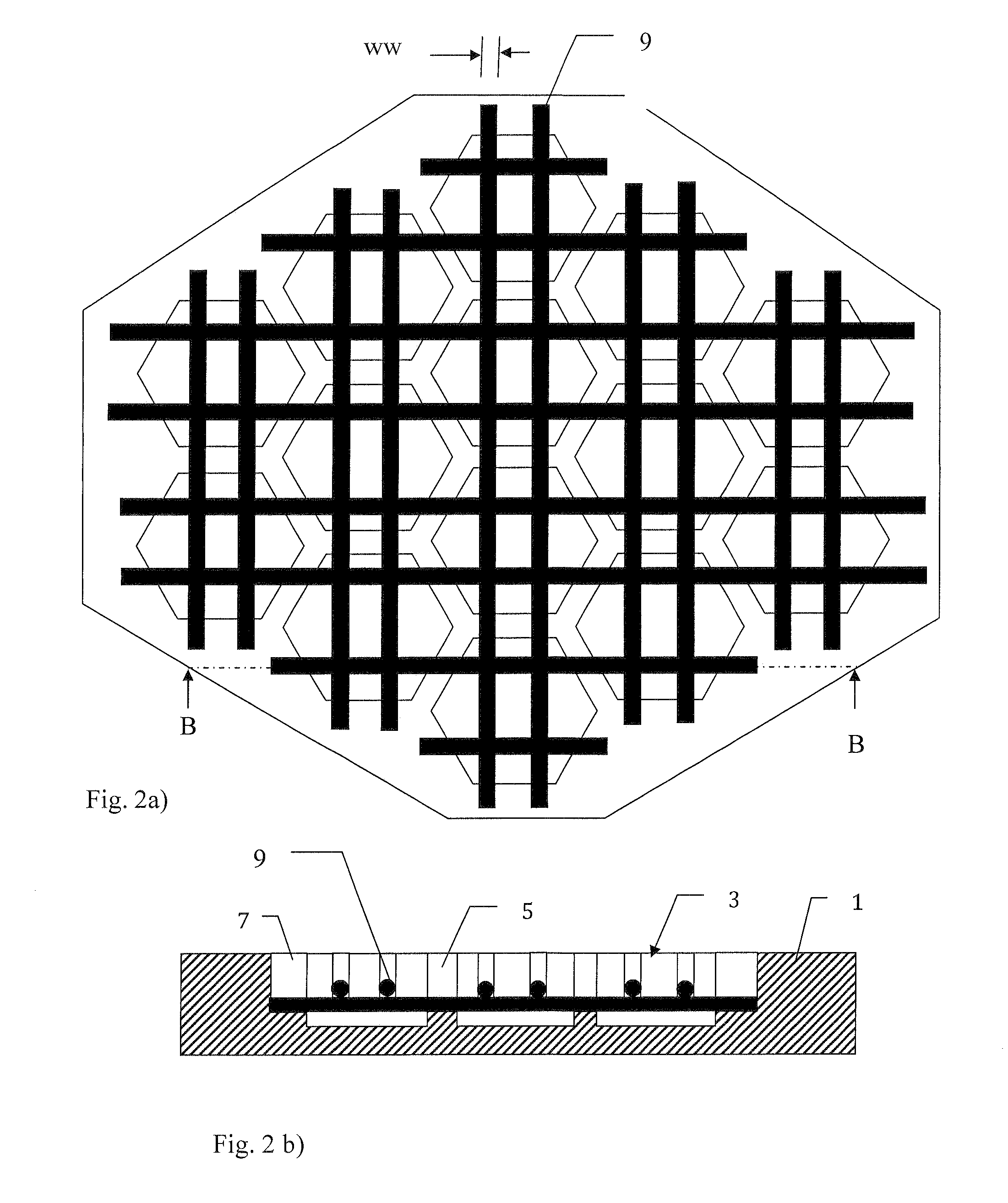
[0065]A mosaic implant was manufactured using the manufacturing method described above using premixed acidic calcium phosphate cement moulded onto Ti wires. The clinical use of this example of a mosaic implant was for the restoration of a large cranial defect. Wires were placed in the mould which was then filled with the premixed acidic calcium phosphate cement and allowed to harden in water for 48 hours at 20 degrees C.
[0066]The premixed acidic calcium phosphate cement consisted of beta-tricalcium phosphate, mono calcium phosphate monohydrate and glycerol. The beta-tricalcium phosphate and mono calcium phosphate monohydrate was mixed in a molar ratio of 1:1 and the glycerol was added to the powder to obtain a powder:liquid ratio of 3.9:1 [g / ml]. The cement was thoroughly mixed until a homogenous paste was formed.
[0067]After hardening the cement was found to consist of mainly the two phases brushite (CaHPO4-2H2O) and monetite (CaHPO4)—however some calcium pyrophosphate (Ca2O7P2) was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com