Automated reporting of payments made to patients for their participation in a clinical study in a blinded manner to the sponsor of the clinical study
a technology of automatic reporting and clinical study participation, applied in the field of automatic reporting of clinical study participation payments to patients in blinded manner to the sponsor of the clinical study, can solve the problems of inability to reproduce the compensation of cash and checks, and the difficulty of obtaining checks,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016]Certain terminology is used herein for convenience only and is not to be taken as a limitation on the present invention.
I. Overview
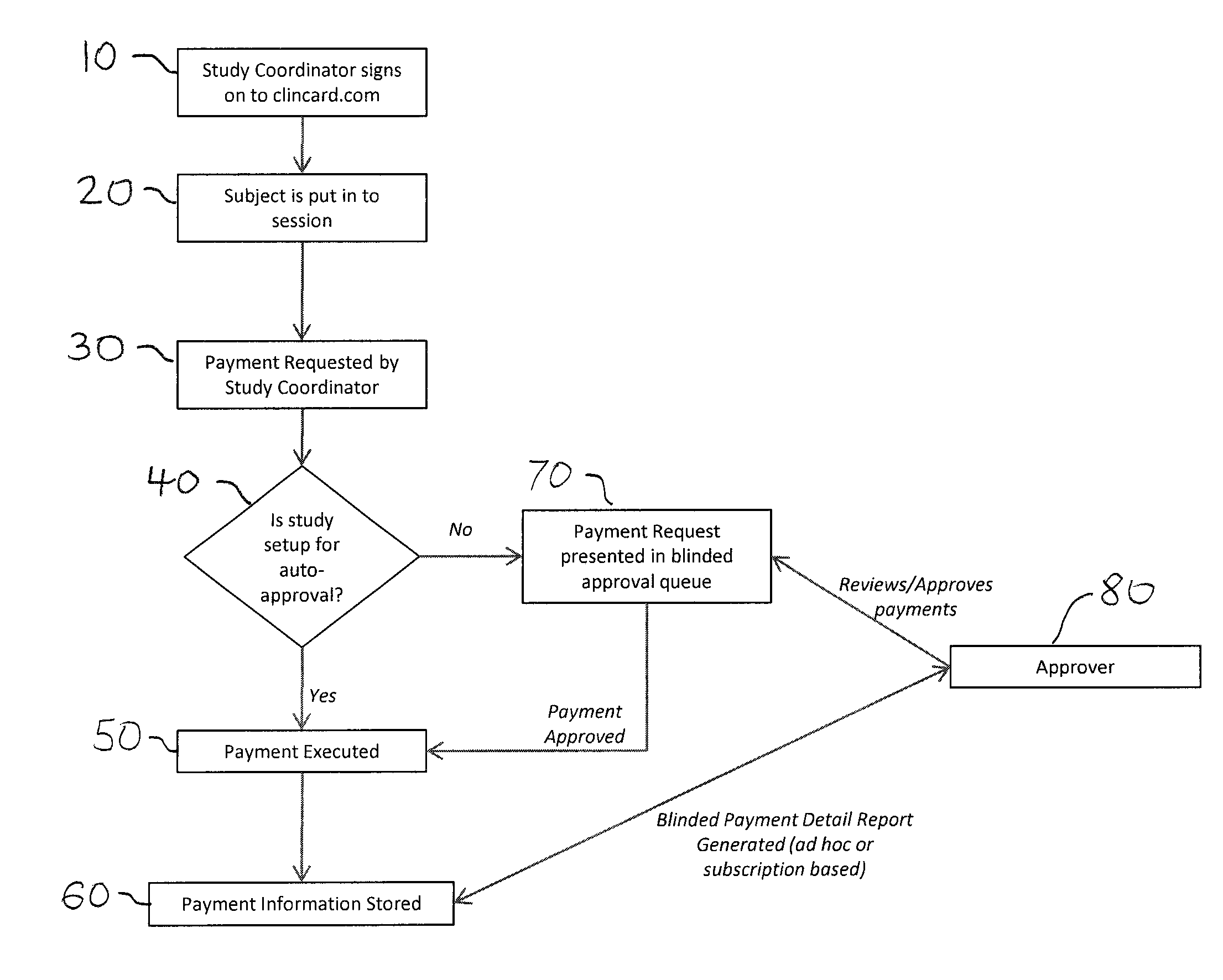
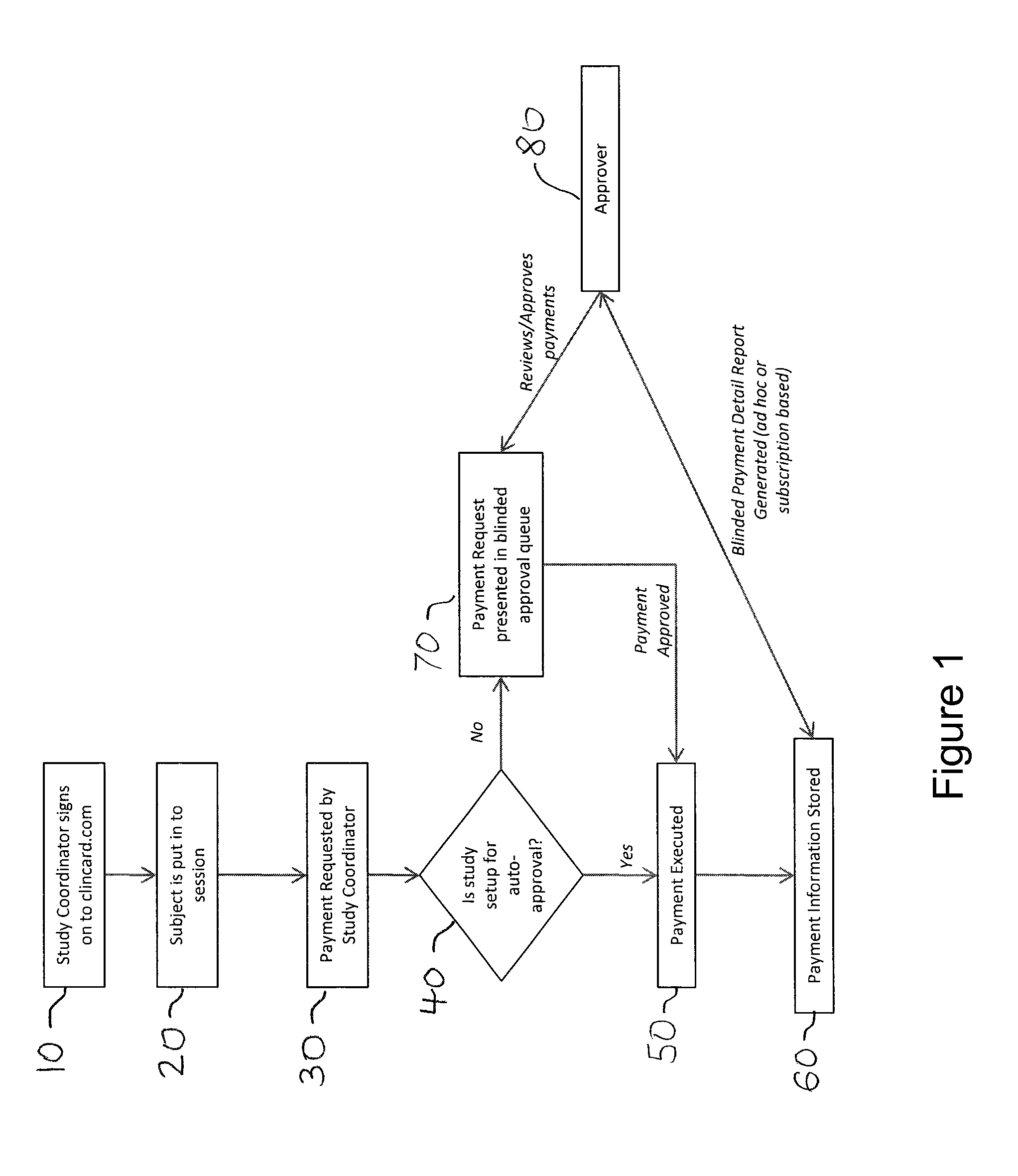
[0017]Developments in payment technology has allowed for the transfer of funds to be provided in a real-time manner via a prepaid debit card, thereby providing the same access to compensation as historic payment methods, while establishing central electronic based records detailing the time, amount, and parties involved in a compensation transaction.
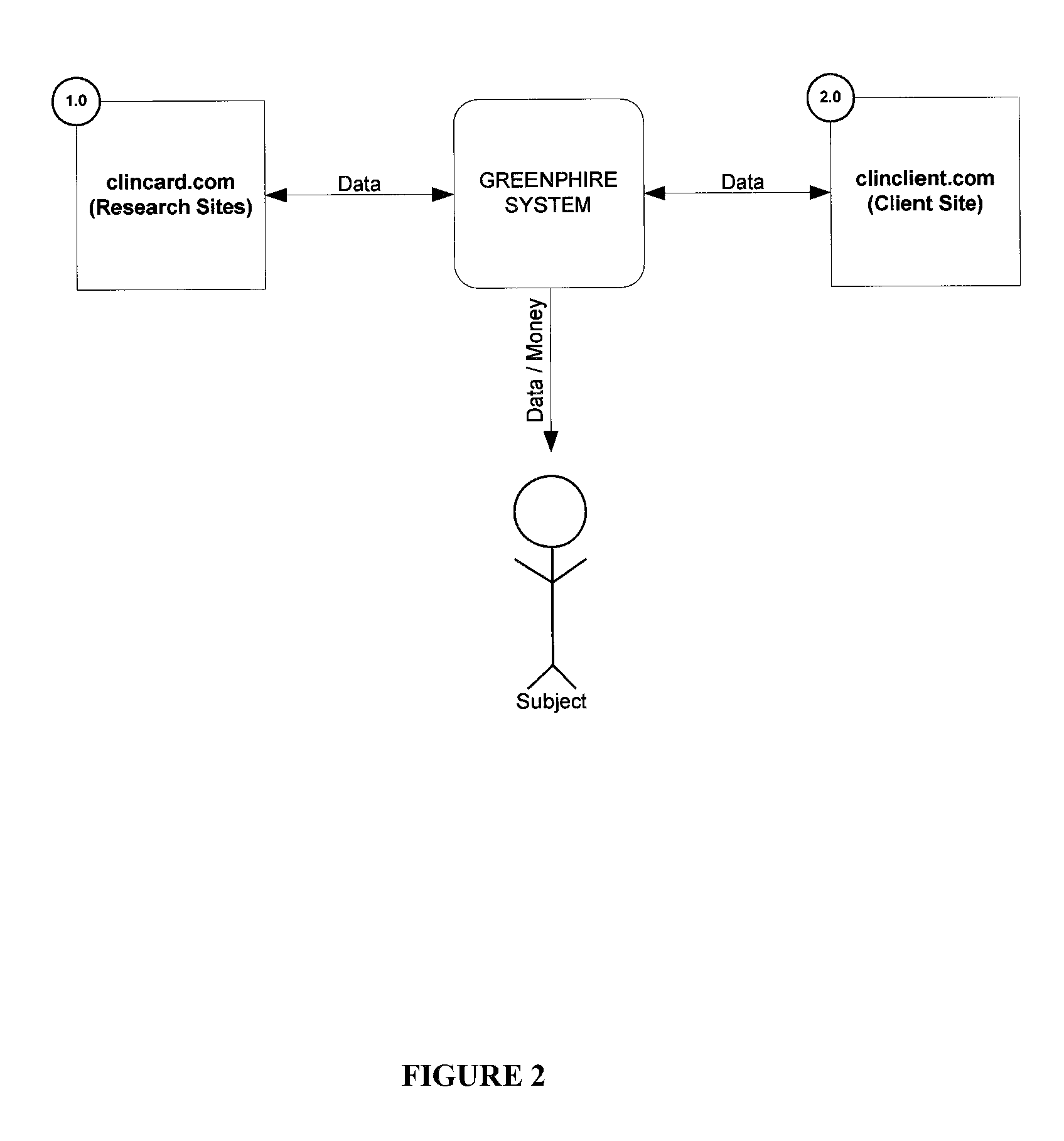
[0018]Greenphire LLC (Greenphire), located in King of Prussia, Pa., is a clinical trials technology company that has created an application which allows parties within the clinical study process to utilize the benefits of the prepaid debit card payment technology to provide subject stipend / reimbursement payment. The process in which Greenphire allows for payment approvals and provides reporting of subject compensation provides an innovative instrument in which study participant compensation information ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com