Monoamine oxidase inhibitors and methods for treatment and diagnosis of prostate cancer
a technology of monoamine oxidase inhibitors and prostate cancer, which is applied in the direction of enzymology, anti-noxious agents, drug compositions, etc., can solve the problems of only partially effective treatments, undesired side effects, and patients' effectiveness, so as to facilitate the switch between modalities, minimize background noise, and maximize sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
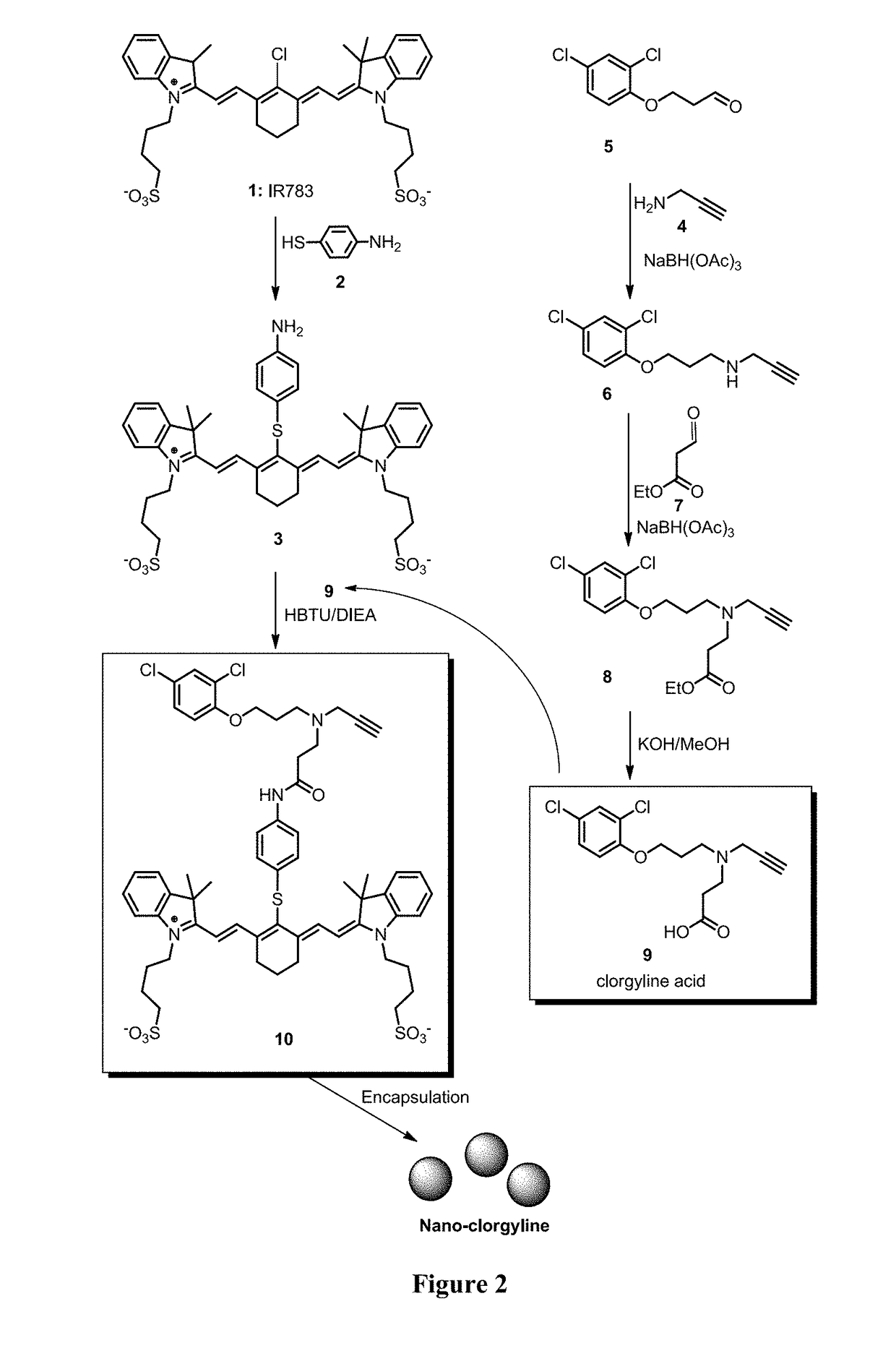
Examples
example 1
MAO-A KO in Host Experiment 1: Murine F9 Teratocarcinoma Tumor Xenograft in WT and MAO-A KO Mice
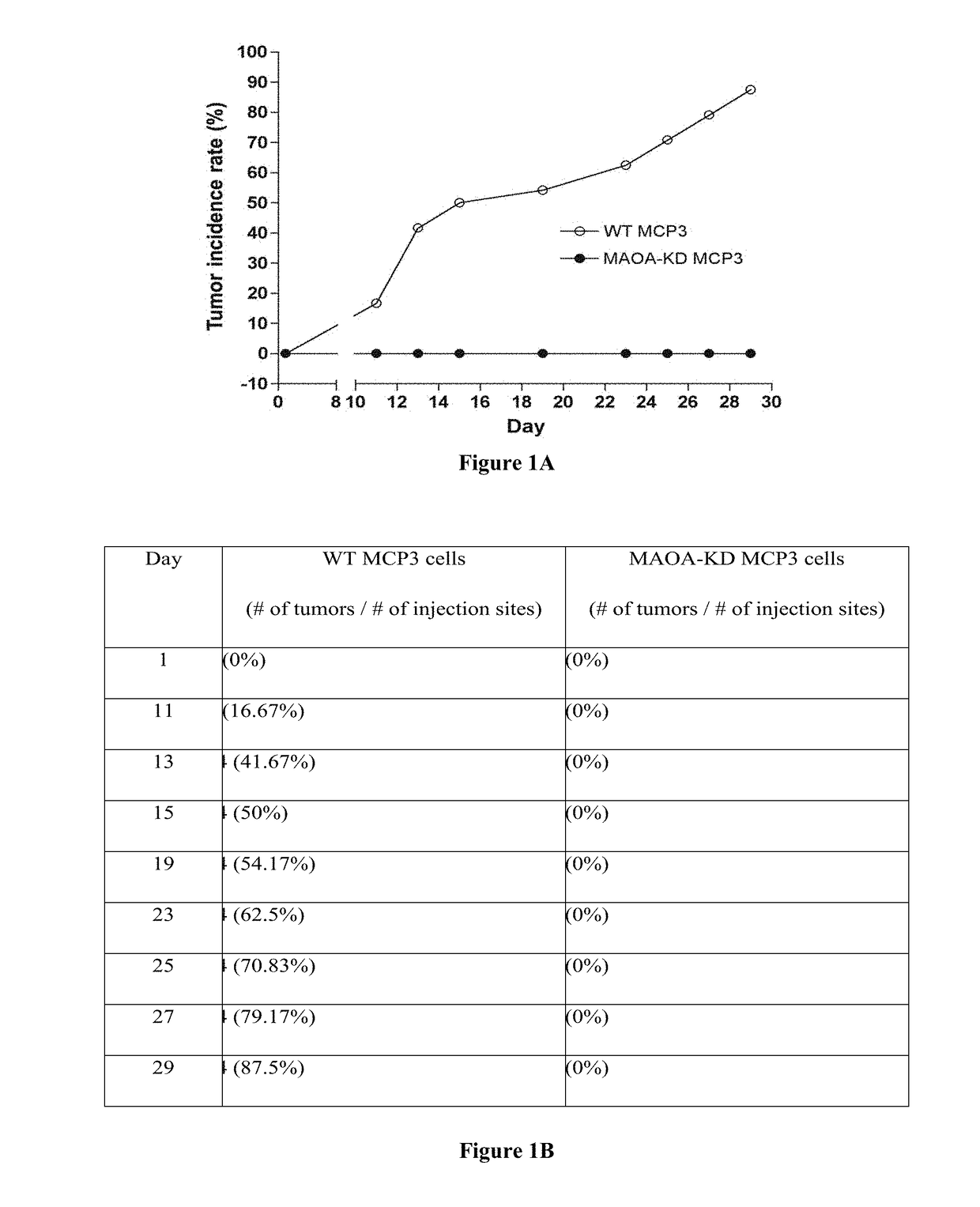
[0129]Cell # injected: 1×105 Mice #: WT (N=9) and MAO-A KO (N=9) Tumor injection site #: WT (2×9=18) and MAO-A KO (2×9=18) Tumor incidence rate: WT (11 / 18=61.1%) and MAO-A KO (3 / 18=16.7%) Tumor growth: WT>MAO-A KO (pMAO-A KO (p<0.05)
example 2
MAO-A KO in Host Experiment 2: Murine MCP3 (pten / p53 Double KO) Prostatic Tumor Xenograft in WT and MAO-A KO Mice
[0130]Cell # injected: 1×106 Mice #: WT (N=3) and MAO-A KO (N=3) Tumor injection site #: WT (4×3=12) and MAO-A KO (4×3=12) Tumor incidence rate: WT (11 / 12=91.7%) and MAO-A KO (10 / 12=83.3%) Tumor growth: WT>MAO-A KO (pMAO-A KO (p=0.25)
example 3
MAO-A KO in Host Experiment 3: Murine MCP3 Prostatic Tumor Xenograft in WT and MAOA KO Mice
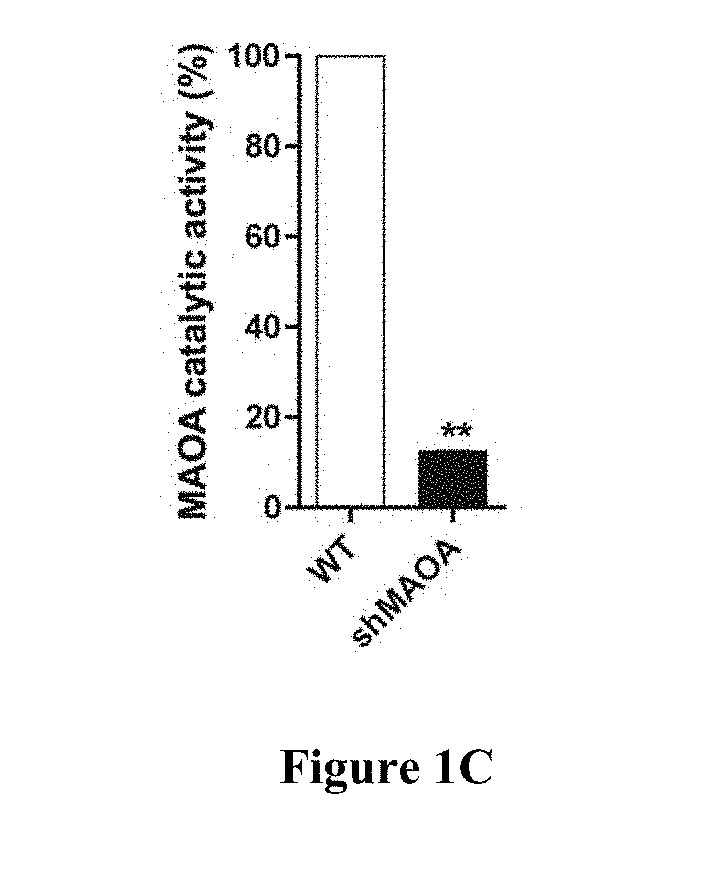
[0131]Cell # injected: 1×105 Mice #: WT (N=4) and MAO-A KO (N=5) Tumor injection site #: WT (3×4=12) and MAO-A KO (3×5=15) Tumor incidence rate: WT (10 / 12=83.3%) and MAO-A KO (0 / 15=0) Tumor growth: No MCP3 tumor growth in MAO-A KO mice
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com